Participants
A total of 22 patients with stable SCZ and 26 demographically matched HCs were recruited. All the patients were on regular antipsychotic medication and stable. All patients with SCZ were recruited from the West China Hospital Mental Health Center of Sichuan University from August 2021 to May 2023. The diagnosis was made by experienced psychiatrists using structured clinical interviews based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), including the Structured Clinical Interview for DSM (SCID)22. The HCs were recruited through posters and website advertisements and filtered using a non-patient version of the clinical definitive interview (Structured Clinical Interview for the DSM-Non patient edition, SCID-NP) to ensure no history of mental illness among HCs and their first- and second-degree relatives. The severity of psychotic symptoms in the participants was assessed using the Positive and Negative Syndrome Scale (PANSS)23 before the MRI scanning. The PANSS was administered to the HCs to ensure that we could differentiate between symptomatology in schizophrenia patients and the absence of symptoms in healthy individuals. To ensure accurate symptom capture, we followed the standardized PANSS administration procedures, and all assessments were conducted by trained clinicians with experience in administering the PANSS. All participants were right-handed. Stabilized individuals with schizophrenia must fulfill specific criteria, demonstrating sustained stability in mental mood and symptoms throughout the experimental phase (i.e., no psychiatric hospital admissions, acute exacerbations, or imprisonments). This entails maintaining a score below 4 for each positive symptom on the PANSS for a minimum duration of 6 months24. All SCZ patients were prescribed second-generation antipsychotic medications according to clinical guidelines. Daily dosages of antipsychotic drugs were converted into chlorpromazine (CPZ) equivalents. Participants were excluded if they1 were pregnant or lactating women2, had a history of any neurologic illnesses, such as brain tumors, epilepsy, and so forth3, exhibited contraindications to MRI, such as claustrophobia or metal-containing implants in the body4, had alcohol and drug dependence or abuse5, were intolerant to prolonged sitting or supine positions during TMS treatment, and6 had incomplete MRI data or poor image quality. Besides, only edited spectra with GABA and Glx fitting error of less than 15% were included in the final analyses to further ensure the robustness of our results25. Three patients with SCZ were excluded because of high fitting errors and slight involuntary head movements during MRI scanning, leaving a final cohort of 19 patients with SCZ and 26 HCs (Table 1). The two groups were sex-matched, but the patients with SCZ were considerably older than the HCs.
The study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. All participants or their guardians were informed of the study details, a full understanding of the study protocol, and signed an informed consent form.
Data acquisition
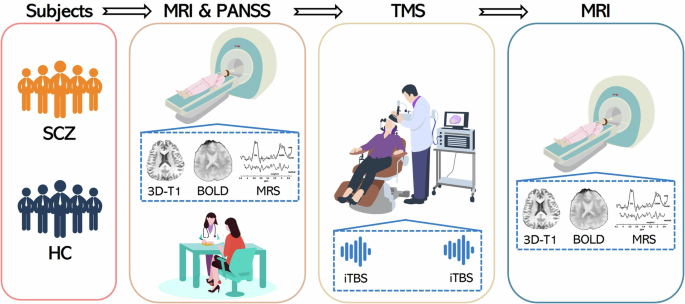
All participants underwent two MRI scans immediately before and after TMS using a 3.0 T MRI scanner (Ingenia Elition, Philips Healthcare, the Netherlands) with a 32-channel head coil at the West China Hospital of Sichuan University (Fig. 1). The structural sequence and parameters were as follows: high-resolution 3-dimensional turbo field echo T1-weighted (3D-T1) images with repetition time/echo time (TR/TE) = 7.8/3.7 ms, slice thickness = 1 mm, sagittal slices = 181, flip angle = 8°, matrix = 256 × 256, field of view = 256 × 256 mm2, and voxel size = 1.0 × 1.0 × 1.0 mm3. High-resolution resting-state blood-oxygen-level-dependent (BOLD) images were acquired using an echo-planar imaging (EPI) sequence with the following parameters: TR/TE = 4000/35 ms, slice thickness = 2.2 mm (no slice gap), slice number = 65, flip angle = 90°, matrix = 144 × 144, field of view = 220 × 220 mm2, voxel size = 1.5 × 1.5 × 2.2 mm3, scan time = 20 min, and total time points = 300 (150 time points with posterior-anterior phase-encoding directions and 150-time points with anterior-posterior phase-encoding directions). The two EPI acquisitions with opposite phase-encoding directions were used to perform EPI distortion correction.
Both schizophrenic patients and healthy controls followed this flow to acquire data. At baseline, MRI scans and PANSS measures were completed. Afterwards, subjects were directed to the TMS treatment room for TMS. The stimulation area was the posterior part of the cerebellum near the midline, and the coil was placed as shown in the figure. Immediately after TMS, subjects underwent another MRI scans. Both MRI scans contained 3D-T1, BOLD and MRS. SCZ schizophrenia; HC healthy controls; 3D-T1 3-dimensional turbo field echo T1-weighted images; BOLD blood-oxygen-level-dependent images; MRS magnetic resonance spectroscopy; TMS transcranial magnetic stimulation; iTBS intermittent theta-burst stimulation.
We used the MEscher-GarwOod point resolved spectroscopy (MEGA-PRESS) sequence26,27 for detecting the cerebellar GABA content, which performed interleaved excitation during the scan28. The MEGA-PRESS sequence parameters were as follows: TR/TE = 2000/68 ms, acquisition bandwidth = 2000 Hz, phase cycles = 8, averages = 144 on and 144 off, and scan duration = 10 min 12 s. The variable power and optimized relaxation delays were used for water suppression. The second-order shimming (pencil beam volume) was used for improved field homogeneity. For each participant, a voxel of interest (VOI) of 30 × 20 × 20 mm3 was placed at the left crus I/II of cerebellum. Given that the MEGA-PRESS sequence imaging time was 10–15 min, and considering the brief immediate effects of TMS, and the “Universal Cerebellar Transform” theory suggests that the cellular structure of the cerebellar cortex is evenly distributed29, we chose to acquire only the left cerebellar MEGA-PRESS sequence to ensure that the BOLD sequence could be effectively collected within the available time frame following TMS. The measured GABA signal using the MEGA-PRESS protocol is commonly referred to as GABA+ with the contribution from macromolecules.
Transcranial magnetic stimulation
After the baseline MRI scan, the participants underwent TMS. Focal magnetic stimulation was applied using the M-100 Ultimate pulsed magnetic field stimulator (Shenzhen Yingchi Technology Co., Ltd, China) and oil-cooled figure-of-eight coils (70-mm outside diameter per wing). We introduced the QuickVision neuro-navigation system (Shenzhen Yingchi Technology Co., Ltd, China) to build the head model using 3D-T1 images for obtaining more accurate target localization and online control of coil positioning.
TMS was first applied to the bilateral M1 to determine the resting motor threshold (MT) as the base reference value for subsequent stimulation. In the single-pulse mode, the bilateral M1 was stimulated separately, and the MT was determined according to the involuntary contractions of the fingers. The final selected threshold was the average of the bilateral MT. Then, the TMS coil was pointed to the middle of the bilateral crus I/II of the posterior cerebellum (Fig. 1 and Figure S1)30. Each participant underwent two sessions of iTBS. The iTBS was specifically chosen due to its excitatory metaplastic effects. Both iTBS sessions were delivered at the same location. The iTBS sequence consisted of 600 pulses applied in a theta-burst pattern (bursts of 3 stimuli at 50 Hz repeated at a 5-Hz frequency) with 20 trains, each lasting 2 s and an intertrain interval of 8 s (total stimulation time of 3 min 12 s), with a stimulation intensity of 80% of MT. Each participant received a total of 1200 pulses across the two sessions, with a minimum interval of 15 min between sessions. Previous studies have demonstrated the safety of delivering 1200 pulses to the cerebellum at 80% of the individual’s MT31,32. The two consecutive iTBS sessions were conducted in order to maximize the immediate effects of cerebellar stimulation and minimize potential confounds related to time delays between sessions.
After completing TMS, participants were immediately guided to the MRI suite for the second scan without delay. To minimize the time interval between the final iTBS session and the MRS scan, only localizer and T1-weighted imaging sequences were performed prior to MRS acquisition, ensuring the interval typically remained under 10 min.
Resting-state functional MRI preprocessing
Before processing the resting-state functional MRI (rs-fMRI), a recon-all needed to be executed on 3D-T1 images using FreeSurfer software (version 6.0, https://surfer.nmr.mgh.harvard.edu/) to segment the cortical structures as a template for subsequent rs-fMRI registration.
The Computational Brain Imaging Group (https://github.com/ThomasYeoLab/CBIG) toolbox was used to preprocess the rs-fMRI data including the slice time correction, motion correction by calculating the framewise displacement (FDrms) and voxel-wise differentiated signal variance (DVARS)33,34 and removing frames by either FDrms > 0.2 or DVARS > 50, spatial distortion correction, nuisance regression, temporal interpolation of censored frames, bandpass filtering at 0.009–0.08 Hz, projections to standard surface (fsaverage 5), and smoothing with a kernel size of 6 mm. For further details, please refer to the supplementary materials.
CTC circuitry construction
After preprocessing, we extracted the mean time series from each of the 270 nodes in the extended Power Atlas as described in a previous study7,35. The mean time series for each of the 270 nodes in the extended Power atlas were extracted from the preprocessed images. Each node represented as a sphere with a 5 mm radius, centered on a specific coordinate point (https://github.com/brainspaces/power264). It is important to note that the original Power atlas included 264 nodes, but excluded regions such as the hippocampus, bilateral amygdala, and bilateral ventral striatum. To ensure complete brain coverage, Cao et al. added these additional nodes36.
For each participant, we constructed a 270 × 270 pairwise whole-brain connectivity matrix by calculating the Pearson correlation coefficient between the extracted time series. A Fisher’s z-transformation was then applied to the functional connectivity matrix to normalize the correlation values. The connectivity strength of the previously reported CTC circuitry was extracted from these connectivity matrices7. The reported CTC network, including 84 links, was mainly concentrated in the thalamus and posterior cerebellum35,37. A detailed list of these edges is provided in Table S1.
MEGA-PRESS preprocessing
The MEGA-PRESS sequences were quantified using the GANNET 3.0 toolkit (http://www.gabamrs.com), a MATLAB-based quantitative batch analysis tool specifically for GABA/Glx-edited MRS28, which required invoking Statistical Parametric Mapping Software 12 (SPM12, http://www.fil.ion.ucl.ac.uk/spm) for segmentation of cerebellar tissue. The metabolite levels were referenced to the unsuppressed water signal, and the GABA and Glx levels were corrected using the proportion of gray matter, white matter, and cerebrospinal fluid (CSF) within the VOI. Additionally, 16 unsuppressed-water signal averages were also acquired as an internal concentration reference. To ensure the accuracy of the cerebellar segmentations, we performed visual quality assurance on each participant’s VOI, specifically checking for correct segmentation.
Furthermore, TMS treatment necessitates the subject to vacate the scanner bed, necessitating the relocation and repositioning of the cerebellar VOI for the post-TMS scan. It is imperative that the two VOIs (pre- and post-TMS) are positioned in approximate alignment to facilitate the comparison of neurotransmitter changes within the VOIs. To achieve this, we computed the overlap rate between the two VOIs. Image registration of the left cerebellar VOIs before and after TMS was conducted using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL, version 6.0, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki)38,39 to ascertain the extent of VOI overlap, thereby determining whether the VOI remained in the same position pre- and post-TMS.
Statistical analysis
Linear Mixed-Effects Model (LMEM) was employed to assess trends in mean CTC connectivity at baseline and post-TMS. Subsequently, two-sample t-tests were conducted to compare mean CTC connectivity between SCZ patients and HCs at both baseline and post-TMS. Sex, age, and CPZ equivalents were included as covariates. Likewise, LMEM and two-sample t-tests were employed to analyze GABA and Glx levels, and Glx/GABA ratio at baseline and post-TMS.
The FCs of the 84 links within the CTC circuitry were compared between SCZ patients and HCs using two-sample t-tests at baseline and post-TMS, adjusting for sex, age, and CPZ equivalents as covariates. Multiple comparisons were corrected using the false discovery rate (FDR) at a significance level of P < 0.05.
First, we calculated the correlations between the PANSS total score and symptom scores with mean CTC connectivity, Glx, GABA, and the Glx/GABA at baseline. Second, we assessed the correlations between mean CTC connectivity and GABA, Glx, and the Glx/GABA ratio at both baseline and after TMS within the SCZ and HC groups, respectively. Additionally, we analyzed the correlation between baseline symptom scores and changes in mean CTC connectivity, GABA, Glx, and the Glx/GABA ratio (from baseline to post-TMS).
Furthermore, to explore the relationship of GABA, Glx, and Glx/GABA with CTC hyperconnectivity in SCZ patients, the magnitude of the mediation effect was calculated at baseline. Mediation analyses were conducted to investigate the role of cerebellar GABA, Glx, and the Glx/GABA ratio as mediators in the relationship between group and mean CTC connectivity. For each mediation analysis, the group was set as the independent variable, with the corresponding neurochemical measure (GABA, Glx, or Glx/GABA) as the mediator, and mean CTC connectivity as the dependent variable. The significance of the indirect effect was tested using bootstrapping with 5000 resamples.
Two-sample t-tests were employed to assess differences in MT values, cerebellar VOI overlap rates, GABA and Glx peak fitting errors, and within-VOI segmentation ratios (including gray matter, white matter, and CSF) between SCZ and HC groups at baseline and post-TMS, with adjustments made for sex and age.
Demographic and clinical data were compared between the two groups using the Pearson chi-square test for sex distribution and the two-sample t-test for age distribution and scale scores. All statistical analyses were conducted using R (version 4.2.1), with significance set at P < 0.05.