Brain activity is shown in gold (artist’s illustration). A newly approved antipsychotic achieves its effects by activating brain proteins called muscarinic receptors.Credit: Sebastian Kaulitzki/Science Photo Library
The first schizophrenia medication in decades with a new mechanism of action won US regulatory approval today. The approval offers the hope of an antipsychotic that would be more effective and better tolerated than current therapies.
The drug, known as KarXT, targets proteins in the brain known as muscarinic receptors, which relay neurotransmitter signals between neurons and other cells. Activating these receptors dampens the release of the chemical dopamine, a nervous-system messenger that is central to the hallmark symptoms of schizophrenia, such as hallucinations and delusions.
Pruning hypothesis comes of age
But muscarinic signalling also modulates other brain circuits involved in cognition and emotional processing. This mode of action provides KarXT with a more comprehensive therapeutic effect than other schizophrenia treatments, which mainly blunt dopamine activity alone.
In clinical trials, KarXT not only alleviated core symptoms of schizophrenia, but also showed signs of improving cognitive function, all while avoiding many of the burdensome side effects commonly associated with older antipsychotics.
“This will be a revolution of the treatment of psychosis, and I’m not saying this lightly,” says Christoph Correll, a psychiatrist at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, who helped to analyse data from the trials. “Now we will now be able to treat people who haven’t been helped with traditional antipsychotics. That’s highly exciting.”
Hope for tailored treatment
KarXT is just the first of many next-generation drug candidates designed to engage muscarinic receptors in the brain. Several follow-on schizophrenia therapies are already in or nearing clinical trials, showing promise for improved tolerability and more convenient dosing schedules.
This progress is leading clinicians and drug developers to imagine a future in which schizophrenia treatment becomes more tailored to individual needs — providing an alternative for the many people who don’t benefit from current therapies or abandon them owing to intolerable side effects.
“This provides an option that is completely outside the toolbox that we have right now,” says Ann Shinn, a psychiatrist at McLean Hospital in Belmont, Massachusetts, who has no commercial ties to KarXT.
From rejection to revival
KarXT traces its roots back to early 1990s, when researchers at Eli Lilly in Indianapolis, Indiana, began developing xanomeline — a muscarinic-activating agent designed mainly to boost memory in people with Alzheimer’s disease, but it has been explored as a potential treatment for schizophrenia, too.
Trials showed that the drug offered both antipsychotic and cognitive benefits1,2. But xanomeline also caused nausea, vomiting and stomach pain — because muscarinic receptors are active in the gut as well as the brain — leading Lilly to ultimately shelve the drug.
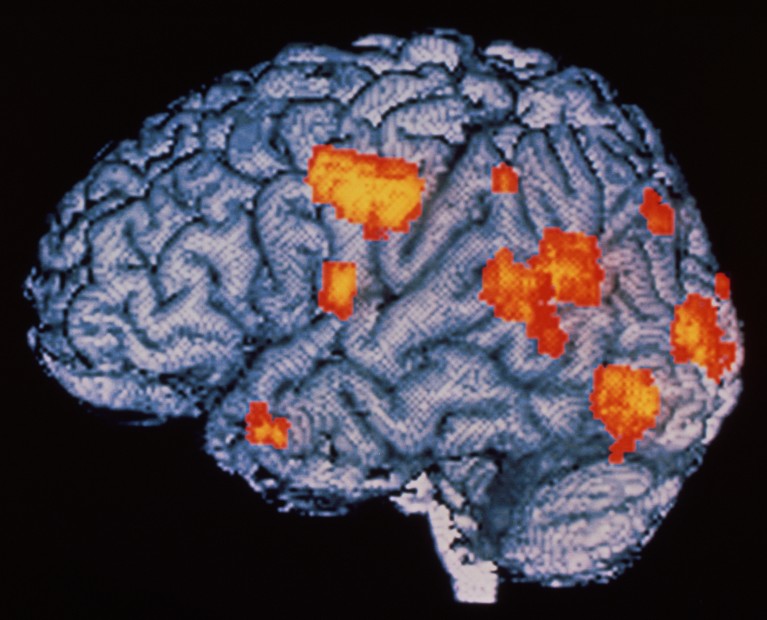
Flashes of activity (red and yellow; artificially coloured) light up the brain of a person having hallucinations caused by schizophrenia.Credit: Wellcome Centre Human Neuroimaging/Science Photo Library
Years later, biotech executive Andrew Miller devised a strategy to revive the therapy. He recognized that administering the muscarinic-activating agent together with another compound that blocks xanomeline’s effects outside the brain could maintain the cognitive and antipsychotic benefits without causing severe gastrointestinal distress.
In 2009, Miller formed a company called Karuna Therapeutics, based in Boston, Massachusetts. Karuna combined xanomeline with a drug called trospium. This well-understood molecule blocks muscarinic receptors and does not cross the blood–brain barrier, meaning that it selectively prevents side effects in the gut without interfering with xanomeline’s action in the brain.
Thus KarXT was born.
In clinical trials, the two-in-one pill outperformed a placebo in relieving characteristic symptoms of schizophrenia3,4, without the weight gain, sedation or movement issues that are commonly associated with existing antipsychotics. The side effects of KarXT were largely limited to gut disturbances, which tended to resolve after a week or two of daily use.
There were also strong signs of cognitive benefit, with preliminary indications5 that KarXT might also help to mitigate symptoms such as blunted affect and lack of motivation. “It’s encouraging,” Stephen Marder, a psychiatrist at the University of California, Los Angeles, says of these ancillary effects. (Marder assisted with some of the analyses). But these effects need to be verified in a “focused study,” he says.
High price
The drug has a few shortcomings. For one, it requires twice-daily administration, and studies indicate that more frequent dosing schedules are linked to higher rates of non-adherence and treatment discontinuation in people with schizophrenia6. “That’s a big limitation,” says Nate Sutera, a psychiatric pharmacist at the University of Nebraska Medical Center in Omaha — particularly because many antipsychotics are now available as long-acting injectables, requiring only a few doses annually.
KarXT also comes with an anticipated price tag of roughly US$20,000 per year7, raising concerns among health economists about its cost-effectiveness compared with alternatives. Despite this, most industry analysts predict strong demand, with peak annual sales projected in the billions. This potential drove Bristol Myers Squibb (BMS) in Princeton, New Jersey, to acquire Karuna for approximately $14 billion this year.

First genomic study of schizophrenia in African people turns up broken genes
Other drug makers are likewise seeing the value of targeting muscarinic receptors, pursuing various strategies to improve on the profile of KarXT. Some are developing formulations with more convenient dosing schedules. Others are focused on greater target selectivity, aiming to design molecules that activate only specific muscarinic receptors — either the M1 receptor, linked to cognitive benefits, or the M4 receptor, which underpins antipsychotic effects, but not both, as KarXT does.
One such drug candidate, an M4-selective agent called emraclidine, seems to offer antipsychotic effects similar to those of KarXT, with improved tolerability, although it offers potentially fewer cognitive benefits, according to early clinical testing8.
Former Karuna chief executive Steven Paul, a psychiatrist now at the Washington University School of Medicine in St. Louis, Missouri, welcomes the wave of innovation in targeting muscarinic signalling that KarXT helped to unleash — and he looks forward to discovering the best ways to harness this therapeutic strategy.
“Now we have new biology and new pharmacology to explore,” he says. “It will be fun and scientifically relevant — and, hopefully, clinically beneficial to patients — to find out.”


