Subgrouping individuals according to their response to antipsychotic treatment has potential for identifying clinically relevant prognostic markers. This could lead to effective personalised treatments which will improve clinical outcomes in individuals with schizophrenia. Personalised approaches could optimize therapeutic interventions, reducing trial-and-error prescribing and enhancing patient quality of life. The multifactorial nature of schizophrenia presents a significant challenge to personalise treatment. The interaction of genetic, environmental, and biological factors complicates the development of accurate predictive models. This complexity requires a multidimensional approach to integrate various data types and analytical methods to address the disorder’s intricacies. To the best of our knowledge, this study marks an early effort to develop a predictive model for categorizing antipsychotic responsiveness.
Model prediction
There have been numerous attempts to develop predictive models for precision psychiatry using ML and deep learning algorithms. However, most are trained on neuroimaging, speech patterns and digital features [21,22,23]. Only a handful were based on molecular biomarker data [8]. The present study was able to achieve a reasonable performance of 0.74 to distinguish people with and without schizophrenia, and 0.88 and 0.78 respectively for differentiating responsiveness to antipsychotics and clozapine. However, the drop in performance for model 3 (differentiating clozapine responsiveness) could be due to small sample size.
Regardless of accuracy and performance, all models still require external validation or practical implementation in clinical settings. The transition from research to clinical application involves addressing challenges to data standardization and integration with electronic health records. Successful implementation could lead to widespread adoption of predictive tools, allowing evidence-based decision-making in psychiatric care. So far, very few prediction tools developed in mental health research have progressed into clinical use, with the prediction of medication response in schizophrenia still in its early stages [24]. Two studies focused on predicting response to medications commonly used in bipolar disorder, namely lithium and quetiapine, utilizing baseline sociodemographic, clinical, and family history information [25, 26]. Although both studies developed models that performed above chance levels, neither model underwent validation in independent samples. In future research, a crucial strategy will be the integration of biological data for comprehensive analyses. Biological markers offer objective measurements of physiological processes or biomarkers, enhancing reliability and reducing variability. Furthermore, the ability to longitudinally monitor changes over time through biological data offers valuable insights into disease progression, treatment efficacy, and individual health trajectories. Therefore, integrating biological data, including molecular biomarkers, offers significant potential to enhance overall model performance and deepen our understanding of the genetic foundations of mental illnesses. This approach could lead to more precise and effective treatments, based on the comprehensive understanding of the biological underpinnings of schizophrenia.
Inflammatory markers
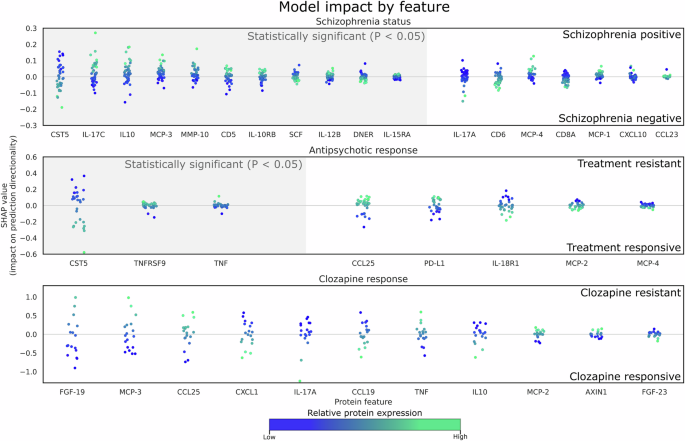
Using ML, we identified 18 proteins to be the most impactful features to differentiate healthy individuals from people with schizophrenia. These investigated proteins were involved in inflammatory processes, such as Jak-STAT, NF-κB, mitogen-activated protein kinases (MAPK), RAS, and TNF signaling pathway, cytokine-cytokine, and receptor interaction pathways [27]. 11 out of these 18 proteins (CD5, CST5, DNER, IL-10, IL-10RB, IL-12B, IL-15RA, IL-17C, MCP-3, MMP-10, and SCF) exhibited significant difference in protein expression levels between healthy individuals and schizophrenia group.
The identification of inflammatory markers associated with schizophrenia supports the growing evidence that immune dysregulation plays an important role in the pathophysiology of the disorder. Results from the present study are concordant with reported findings – higher protein expression levels of commonly studied markers such as CCL23, IL-6, IL-8, IL-10, IL-12, IL-15, TNF and TGF are observed in individuals with schizophrenia when compared to healthy individuals [28, 29]. As chemokines, growth factors, cell surface receptors traditionally, received less attention than cytokines, reports of such group of inflammatory markers in psychosis or schizophrenia are limited and often inconsistent. Nonetheless, we observed a general upward trend for similar markers for individuals with schizophrenia in the present study [30, 31]. The observed elevated levels of cytokines and chemokines indicate a possible ongoing inflammatory state, contributing to neuroinflammation and subsequent neuronal dysfunction observed in schizophrenia [32]. Understanding these biological changes allows new avenues for exploring targeted anti-inflammatory treatments as adjunctive therapies for schizophrenia.
Several meta-analyses on associations between immunological markers and antipsychotics response were limited to pre- and post-treatment in first episode psychosis [33, 34]. Among numerous immune biomarkers studied, IL-1β, IL-6, and TGF-β were consistently reported to have significant reductions post-antipsychotic treatment. Thus far, only one meta-analysis conducted to evaluate the immune alterations induced by clozapine which reported a down-regulation of IL-6 levels in patients who received clozapine, compared to other antipsychotics [35]. Although the present study had similar down-regulation of IL-6 (log2 fold change of −0.112) in TRS compared to ARE, this fold change did not meet statistical significance. Out of the 8 immune markers identified via ML, only IL-18 and TNF from this study was associated with response to amisulpride, risperidone, olanzapine, and/or quetiapine [34]. Further investigation into the role of less-studied inflammatory markers, such as CCL25, CST5, MCP-2, MCP-4, PD-L1, and TNFRSF9 is necessary.
The value of ML for classifying pharmacological subtypes
ML methods such as the Support Vector Machine (SVM) used in this study can discern subtle signals that may not be identified through traditional statistical testing. Unlike conventional statistical methods like the t-tests, ML methods can model multiple features simultaneously and capture relationships between features, which might be overlooked. ML models can handle multidimensional analyses, uncovering intricate interactions, providing a more comprehensive understanding of schizophrenia’s biological landscape.
The SVM was selected for its simplicity, robustness, and its reliance on a good feature set. When coupled with the explainable AI approach SHAP, we were able to improve model interpretability. Based on Fig. 3, several statistically significant features had less predictive power compared to features that were not statistically significant based on the mean absolute SHAP value. Hence, this approach can be powerful for biomarker screening. Contemporary methods like ensemble methods (such as bagging and boosting) are recognised for their superior prediction performance score and popularity. However, in this context, they may not be appropriate due to our limited sample and feature size.
Each datapoint represents a prediction from the test set, the x-axis represents selected proteins, and the y-axis represents the respective impact on the prediction outcome by the model (e.g. towards treatment resistance or treatment positive). Since the datapoints represent the contribution of each sample by each feature to its prediction, they are color coded to its relative protein expression value in the test set. Grey hue along the x-axis denotes proteins that are deemed to be statistically significant. There are no statistically significant features detected between clozapine responsive and clozapine resistant samples. (top) HCL from participants with schizophrenia, (middle) Antipsychotic responsiveness (ARE vs TRS), and (bottom) Individuals with TRS (CRE vs. CRT). Each datapoint represents a sample of each feature, which represents its SHAP value and how it contributes to the final prediction outcome (refer to Supplementary S3 for a comprehensive list of fold changes, P and SHAP values).
Strengths and limitations
Strengths. The sample population is from a single site in a tertiary healthcare institute, which enabled enrollment of a well-characterised clinical sample and reduce heterogeneity in data and sample collection. Adopting plasma proteins enables high throughout analysis, allowing for the simultaneous identification and measurement of a vast array of proteins.
Limitations. The inflammation panel in the current study is a general list and not tailored for psychosis or mental disorders. We were unable to enroll a drug-naïve group to investigate the impact of medication exposure on peripheral immune profiles. Our study lacked longitudinal evaluation, essential for understanding the consistency of immune profiles among pharmacological subtypes, as well as the influence of factors such as exposure to infectious agents or alterations in medications or treatment regimes.
We demonstrated the efficacy of using ML to predict pharmacological subtypes of schizophrenia based on blood-based inflammatory biomarkers. Our study shows that extracted biomarkers from ML models exhibit different expression levels but do not necessarily attain statistical significance possibly due to limited sample sizes and clinical heterogeneity. Despite these limitations, the functional characterization of ML selected biomarkers is supported by contemporary literature, which validates their associations with biological pathways or processes that are relevant to schizophrenia. Hence, ML-based models can be useful for predicting treatment outcomes and yield meaningful markers for understanding underlying mechanisms.