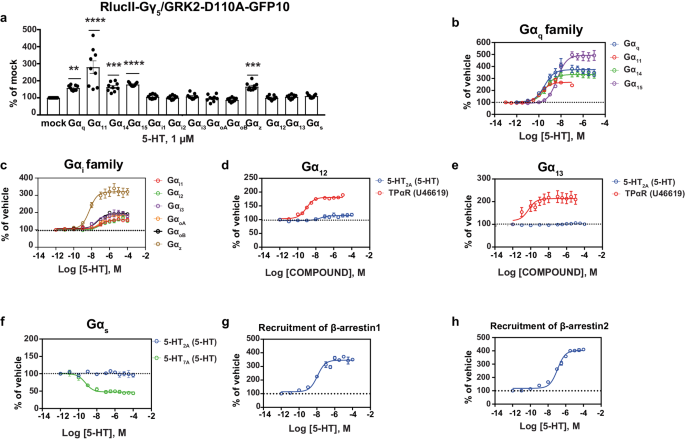
Signaling profile of 5-HT on the 5-HT2AR
As a primary step on this research, we examined the whole G protein-activation profile of 5-HT on the predominantly Gαq-coupled 5-HT2AR in human embryonic kidney (HEK) 293 cells utilizing a BRET2 biosensor to quickly decide the G protein subtypes engaged by a receptor-ligand pair. This biosensor, as beforehand described [30, 42], permits screening for the activation of a whole panel of wild-type Gα subunits belonging to the totally different households; Gαq (Gq, G11, G14, G15), Gαi/o/z (Gi1, Gi2, Gi3, GoA, GoB, Gz), Gα12/13 (G12, G13) and Gαs. The assay relies on the detection of BRET between a GRK2 assemble fused to the blue-shifted inexperienced fluorescent protein GFP10 (GRK2-GFP10) and Gγ5 fused to luciferase from Renilla reniformis (RlucII-Gγ5). The rise in BRET upon G protein-activation displays the dissociation of Gα from Gγ thus permitting an affiliation between the launched RlucII-Gγ5 and GRK2-GFP10. We used a mutant type of GRK2 (D110A), which lacks the RGS area for Gαq, thus eliminating the opportunity of a possible bias in direction of the detection of Gαq activation. Upon stimulation with 5-HT we noticed strong activation of all members of the Gαq household (Gαq, Gα11, Gα14, Gα15) together with Gαz from the Gαi/o/z household (Fig. 1a). The outcomes are corresponding to these obtained utilizing the wild-type (WT) type of GRK2-GFP10 (Supplementary Fig. 1a). Though this sample of activation by 5-HT is in settlement with a current research demonstrating the activation of primarily the Gαq household and Gαz with little or no activation of different Gαi/o relations [43], the 5-HT2AR has additionally been reported to activate pathways downstream of the PTX-sensitive Gαi/o household, particularly with respect to the pathophysiology of schizophrenia and the mechanism of motion of hallucinogenic medication [15, 20, 44, 45]. Particularly, inverse agonist exercise at Gαi1 of the 5-HT2AR-selective ligand (pimavanserin) was just lately reported in human postmortem mind samples and mice mind cortices [26].
a The whole G protein-activation profile of 5-HT (1 µM; 15 min) in HEK293 cells heterologously expressing the untagged 5-HT2AR and the biosensor (RlucII-Gγ5/GRK2-D110A-GFP10, Gβ1 and the respective Gα subunits). Outcomes are expressed as BRET ratio (GFP10/RlucII) as % of mock situation (within the absence of heterologously expressed Gα subunit) (imply ± SEM; n = 3; one-way ANOVA adopted by Dunnett’s put up hoc: **p = 0.0029, ***p = 0.0009, and ****p < 0.0001 in comparison with the mock situation). Focus response curves displaying the activation of the Gαq household (b), Gαi/o/z household (c), Gα12 (d), Gα13 (e), Gαs (f), in addition to the recruitment of β-arrestin1 (g) or β-arrestin2 (h) utilizing the ebBRET-based EMTA biosensor in HEK293 cells heterologously expressing the untagged 5-HT2AR, the next biosensors (rGFP-CAAX together with p63-RlucII for Gαq, Rap1GAP-RlucII for Gαi/o/z, PDZ-RlucII for Gα12 and Gα13, Gαs67-RlucII for Gαs, β-arrestin1-RlucII or β-arrestin2-RlucII for β-arrestins), the respective Gα subunits (Gαq household, Gαi/o/z household, Gα12/13), Gβ1 and Gγ1 (Gαs) or WT-GRK2 (β-arrestins). The HA-TPα receptor (U46619) was used because the constructive management for Gα12/13 whereas the 5-HT7A receptor (5-HT) was used because the constructive management for Gαs. Outcomes are expressed as BRET ratio (rGFP/RlucII), as % over automobile (imply ± SEM; n = 3).
Therefore, we additional investigated the activation of the Gαi/o/z household utilizing the extra delicate EMTA biosensors that measure the rise in BRET sign upon the recruitment of Gα family-specific effectors to the plasma membrane thus being a extra direct reporter of the Gα activation state [20]. The assay used the Gα subunit selective effectors p63-RhoGEF for the Gαq household, PDZ-RhoGEF for Gα12/13 and Rap1GAP for the Gαi/o/z household in addition to Gαs itself fused to RlucII because the vitality donor. The BRET between these donors and the Renilla reniformis GFP (rGFP) anchored on the inside face of the plasma membrane by the CAAX motif from Kras [20] is then used as an indicator of the G protein subtype activation. Much like what was detected with the GRK2-based biosensor, we noticed strong activation of the Gαq relations and Gαz (Fig. 1b, c). Furthermore, we detected activation of the Gαi/o subtypes (Fig. 1c), and this response was discovered to be delicate to pertussis toxin (PTX), suggesting direct activation of the totally different Gαi/o subunits by the 5-HT2AR (Supplementary Fig. 1b). It’s noteworthy that the exercise detected within the absence of heterologous expression of Gα subunit was considerably diminished by PTX, reflecting the activation of endogenously expressed Gαi/o. The remaining response noticed within the presence of PTX more than likely displays activation of the endogenously expressed PTX- insensitive Gαz, according to the strong response noticed utilizing both the GRK2- or Rap1GAP-based biosensors upon heterologous expression of Gαz. Provided that solely weak coupling of 5-HT2AR to Gαz vs different Gαi/o relations had been beforehand reported [43], we in contrast the residual Gαz-mediated response following PTX therapy in three totally different HEK293 cell clones. As could be seen in Supplementary Fig. 1b, the PTX-resistant response represented between 40 ± 8 and 61 ± 7% of the worldwide Rap1GAP response, indicating a cell type-dependent variation within the extent of Gαz coupling which will end result from totally different relative expression of the Gαi/o/z subtypes in several clones. It must also be famous that Kim et al. used a BRET biosensor primarily based on the dissociation between Gα and Gβγ subunits that, in distinction to the EMTA sensors used herein, requires modification of the Gα with the insertion of the vitality donor Rluc within the Gα construction which can have an effect on coupling and makes comparability between totally different Gα coupling troublesome.
The activation of the Gαq and Gαi/o/z household noticed in our research was totally 5-HT2AR-dependent since no responses have been detected within the absence of 5-HT2AR heterologous expression (Supplementary Fig. 1c). To exclude the chance that 5-HT originating from the serum could intervene with the responses, experiments monitoring Gαq activation have been carried out in cells grown in media containing both 2% or 10% new calf serum (NCS). As proven in Supplementary Fig. 1d, no variations have been noticed, indicating that any probably residual 5-HT didn’t intervene with the assay.
With the EMTA biosensor for Gα12 and Gα13 we affirm that the 5-HT2AR doesn’t straight activate Gα12 or Gα13 (Fig. 1d, e), in distinction to the strong activation noticed for the TPα receptor used as a constructive management. Though earlier research have implicated Gα12/13 within the activation of the phospholipase A2 (PLA2) pathway downstream of the 5-HT2AR, different research have additionally proven that there isn’t any proof for a direct coupling of those G proteins by the 5-HT2AR [20, 43, 46]. No activation of Gαs (indicated by a lower in BRET sign on account of Gαs67-RlucII leaving the plasma membrane adorned with the BRET acceptor rGFP as noticed for 5-HT7AR used as a constructive management) was noticed for the 5-HT2AR upon 5-HT stimulation (Fig. 1f). In distinction to beforehand reported 5-HT-promoted cAMP accumulation in untransfected HEK293 cells [47], presumably because of the presence of endogenously expressed 5-HT7AR, we didn’t detect any 5-HT-promoted activation of Gαs in untransfected cells (Supplementary Fig. 1e). This distinction between the 2 research could come up from totally different HEK293 clones getting used or totally different sensitivities of the biosensors. In any case, no coupling of 5-HT2AR to Gαs may very well be detected and this pathway was not additional explored.
Activation of the 5-HT2AR by 5-HT results in the recruitment of the β-arrestins as seen with the strong sign noticed with the EMTA biosensors (β-arrestin1 and β-arrestin2 respectively fused to RlucII recruited to the plasma membrane adorned with rGFP) (Fig. 1g, h). The efficiency of 5-HT (logEC50 and logEC80) for the activation of the totally different G protein subtypes and β-arrestin recruitment is introduced in Supplementary Desk 1.
Useful selectivity profile of antipsychotic medication on the signaling pathways activated by 5-HT
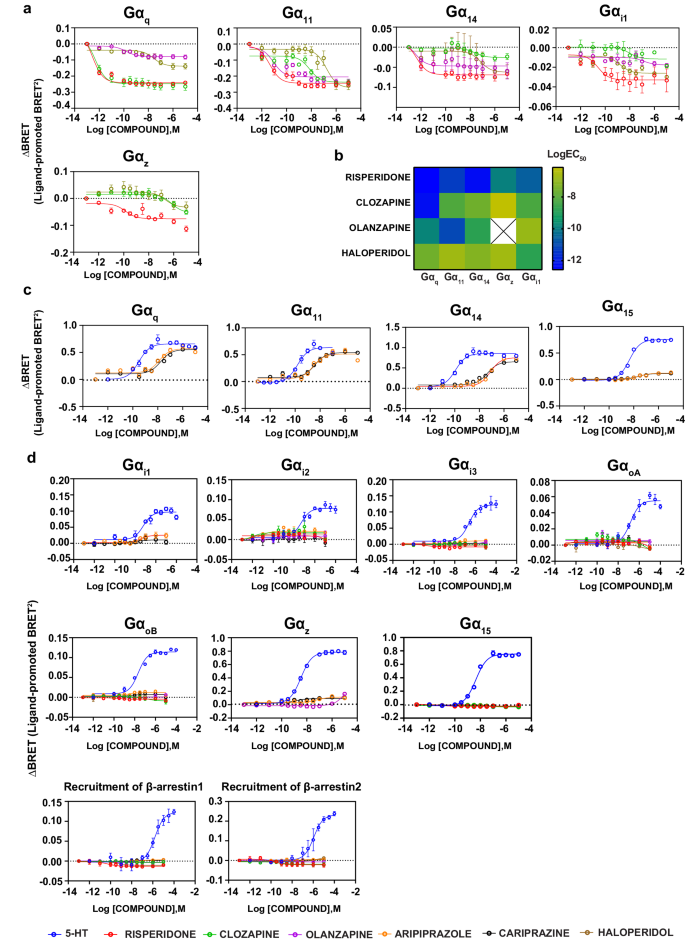
Based mostly on the signaling profile of 5-HT, we examined six antipsychotic medication (risperidone, clozapine, olanzapine, aripiprazole, cariprazine and haloperidol) for his or her exercise on the Gαq and Gαi/o/z relations and for the recruitment of the β-arrestins. When examined within the agonist mode, every antipsychotic has a particular activation/inactivation profile, offering a singular signaling signature, i.e., pathway-preference profile, of their exercise on the 5-HT2AR (Fig. 2). Our analyses reveal that the compounds could be divided into two teams, 4 having inverse agonist exercise (risperidone, clozapine, olanzapine and haloperidol) and two being partial agonists (aripiprazole and cariprazine) on totally different pathways.
a Focus response curves depicting the inverse agonist actions of risperidone, clozapine, olanzapine and haloperidol utilizing the ebBRET-based EMTA biosensors in HEK293 cells heterologously expressing the untagged 5-HT2AR, the biosensor (rGFP-CAAX together with p63-RlucII for the Gαq household and Rap1GAP-RlucII for Gαi1/z) and the respective Gα subunits. b Heatmap illustrating the efficiency (logEC50) of inverse agonist exercise proven by risperidone, clozapine, olanzapine and haloperidol from the focus response curves. The empty cell with a cross signifies no inverse agonist exercise. c Focus response curves depicting the partial agonist actions of aripiprazole and cariprazine on the Gαq household utilizing the ebBRET-based EMTA biosensors in HEK293 cells heterologously expressing the untagged 5-HT2AR, the biosensor (rGFP-CAAX together with p63-RlucII for the Gαq household). d Curves of the six antipsychotic medication at pathways the place there was no activation when examined within the agonist mode utilizing the ebBRET-based EMTA biosensors in HEK293 cells overexpressing the untagged 5-HT2AR, the biosensors (rGFP-CAAX together with p63-RlucII for the Gαq household, Rap1GAP-RlucII for Gαi/o/z household, β-arrestin-RlucII/WT-GRK2 for β-arrestins) and the respective Gα subunits (for Gαq and Gαi/o/z households). Outcomes are expressed as ΔBRET (ligand-promoted BRET) (imply ± SEM; n = 3).
Risperidone, clozapine, olanzapine and haloperidol show pathway desire of their inverse agonist exercise
Though antipsychotics have been reported to have inverse agonist exercise on the 5-HT2AR [29, 48], to our information, that is the primary research assessing this exercise for a set of antipsychotics in any respect the G protein subtypes engaged by this receptor. Total, risperidone, clozapine, olanzapine and haloperidol have been discovered to be inverse agonists (i.e.: inhibiting the constitutive exercise of the 5-HT2AR) primarily at Gαq, Gα11, Gα14, Gαz, and Gαi1. No inverse agonist exercise was detectable towards the opposite Gαi/o relations (Gi2, Gi3, GoA, GoB) or β-arrestin.
Among the many 4 antipsychotics with inverse agonist exercise, risperidone has the best efficiency throughout totally different pathways (sub-picomolar and picomolar vary at Gαq, Gα11 and Gα14; Fig. 2b and Supplementary Desk 2). Whereas risperidone confirmed comparable efficiency on the 5 pathways at which inverse agonism was noticed, there have been main variations within the inverse agonist efficiency of clozapine for the totally different G protein subtypes. It has the best efficiency at Gαq (sub-picomolar; logEC50: −12.31 ± 0.21) adopted by Gαi1 (logEC50: −8.24 ± 0.70), Gα11 (logEC50: −8.16 ± 0.19), Gα14 (logEC50: −7.45 ± 0.30) and at last Gαz (logEC50: −6.17 ± 0.29) (Fig. 2b and Supplementary Desk 2). Of discover, the structurally-related antipsychotics clozapine and olanzapine differ considerably of their inverse agonist efficiency, which probably displays the beforehand reported variations within the binding mode of those medication with respect to the 2 serine residues in TM5 [49]. Whereas the rank order of efficiency for clozapine is Gαq (logEC50: -12.31 ± 0.21) ≫ Gαi1 (logEC50: −8.24 ± 0.70) = Gα11 (logEC50: −8.16 ± 0.19) > Gα14 (logEC50: −7.45 ± 0.30) > Gαz (logEC50: −6.17 ± 0.29), it’s Gα11 (logEC50: −11.06 ± 0.25) ≫ Gαq (logEC50: −9.81 ± 0.22) > Gα14 (logEC50: −8.35 ± 0.34) ≫ Gαi1 (logEC50: −6.87 ± 0.81) ≫ Gαz (no exercise) for olanzapine (Fig. 2b and Supplementary Desk 2). Haloperidol, recognized primarily for its exercise on the dopamine D2 receptor, has the bottom general efficiency among the many 4 antipsychotics displaying inverse agonist exercise on the 5-HT2AR. It confirmed its highest efficiency at Gαi1 (logEC50: −8.77 ± 0.88) and no desire (logEC50: −6.70 to −7.58) among the many different 4 G proteins engaged (Gαq, Gα11, Gα14 and Gαz) (Fig. 2b and Supplementary Desk 2).
Of curiosity, the relative inverse efficacy of the 4 compounds various relying on the G protein subtype thought of. Whereas haloperidol was probably the most efficacious compound for Gα11 and Gα14, probably the most efficacious one for Gαq was risperidone. The three compounds (risperidone, clozapine and haloperidol) with inverse agonist exercise at Gαz have been discovered to be equi-efficacious at this pathway (Supplementary Fig. 2). Taken collectively, the information unravel clear variations amongst clinically used antipsychotic to behave as inverse agonists and displaying distinct G protein selectivity of their inverse efficiency and efficacy.
Along with these compounds we additionally examined a clinically used antipsychotic for psychosis related to Parkinson’s illness, pimavanserin. As proven in Supplementary Fig. 3, much like risperidone, clozapine, olanzapine and haloperidol (Fig. 2), pimavanserin confirmed a sturdy inverse agonist efficacy on Gαq/11 relations. For the Gαi/o/z pathways, it confirmed a weak inverse efficacy for Gαi1 however no different member of the Gαi/o/z household.
The partial agonist exercise of aripiprazole and cariprazine
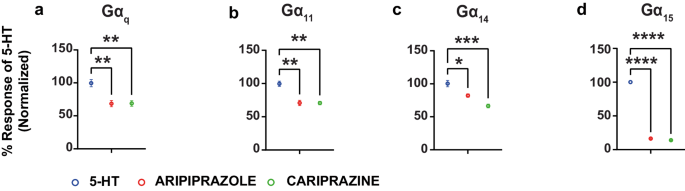
Aripiprazole and cariprazine show purposeful selectivity of their sample of activation of the totally different G proteins. When contemplating the Gαq/11 relations, the 2 compounds confirmed partial agonist exercise with comparatively excessive efficacies at Gαq, Gα11 and Gα14 (their efficacies being between 67.74 ± 4.97 and 73.28 ± 3.61% of that of 5-HT) however solely low efficacy partial agonists at Gα15 (their efficacies being solely 16.29 ± 0.71 and 13.93 ± 0.53% of that of 5-HT; Fig. 2c). The 2 compounds have been discovered to have related partial agonistic exercise (efficiency and efficacy) at every of the Gαq household subtypes (Fig. 3 and Supplementary Desk 3).
Graphs evaluating the maximal response of aripiprazole and cariprazine with that of 5-HT at Gαq (a), Gα11 (b), Gα14 (c) and Gα15 (d) (imply ± SEM; n = 3; one-way ANOVA adopted by Dunnett’s put up hoc check). **p = 0.0046 for aripiprazole and **p = 0.0047 for cariprazine in a, **p = 0.0023 in b, *p = 0.0169, and ***p = 0.0007 for c and ****p < 0.0001 for d, in comparison with the maximal response by 5-HT on the respective pathways.
At Gαi/o/z, very weak but statistically vital activation was detected for aripiprazole solely at Gαi1 and Gαz (Supplementary Figs. 4 and 5). Cariprazine for its half didn’t present statistically vital activation on the Gαi/o/z household (Supplementary Figs. 4 and 5). Lastly, aripiprazole confirmed very weak but statistically vital recruitment of β-arrestin1 whereas cariprazine didn’t (Supplementary Figs. 4 and 5 and Supplementary Desk 3). Collectively, the information present that the 2 compounds have a preferential partial agonistic exercise at Gαq vs Gαi. Differential agonistic exercise was additionally noticed towards members of the identical Gα protein subtype, with Gαq, Gα11 and Gα14 being most popular vs Gα15 for the Gαq/11 household and Gαi1 and Gαz being most popular vs all different Gαi/o/z relations. This desire for particular G protein subtypes is reminiscent to the one noticed for the 4 compounds that confirmed inverse agonistic exercise (Fig. 2a), indicating that this selectivity is linked to the coupling desire of the receptor and never agonist-promoted pathway preferences of the ligands themselves. But the distinction between inverse agonism and partial agonism at these G proteins between risperidone, clozapine, olanzapine and haloperidol on the one hand and aripiprazole or cariprazine on the opposite clearly factors to a distinction within the ligand-promoted regulation of subsets of G protein subtypes between these two teams of antipsychotics.
To evaluate whether or not this distinction in inverse agonism and partial agonism between antipsychotics may translate in in vivo settings, we measured the degrees of D-myo-inositol 1 monophosphate (IP1) within the frontal pole of the cortical lobe dissected from mice handled with risperidone, or aripiprazole or the automobile alone for 60 min. As proven in Supplementary Fig. 6, aripiprazole promoted a statistically vital 10.5 ± 1.6% improve in IP1 ranges in comparison with the automobile situation, according to the partial agonism towards Gαq that we noticed within the cell-based assays. Risperidone therapy, for its half, led to a major 9.2 ± 1.2% discount in IP1 according to the inverse efficacy noticed in cells. Nonetheless, one can not exclude the chance that this decreases outcomes from that antagonistic motion of the drug inhibiting the activation ensuing from endogenous 5-HT as ascertaining inverse agonism in vivo is at all times hampered by the attainable tonic presence of endogenous agonists.
Exercise of the antipsychotics examined in antagonist mode
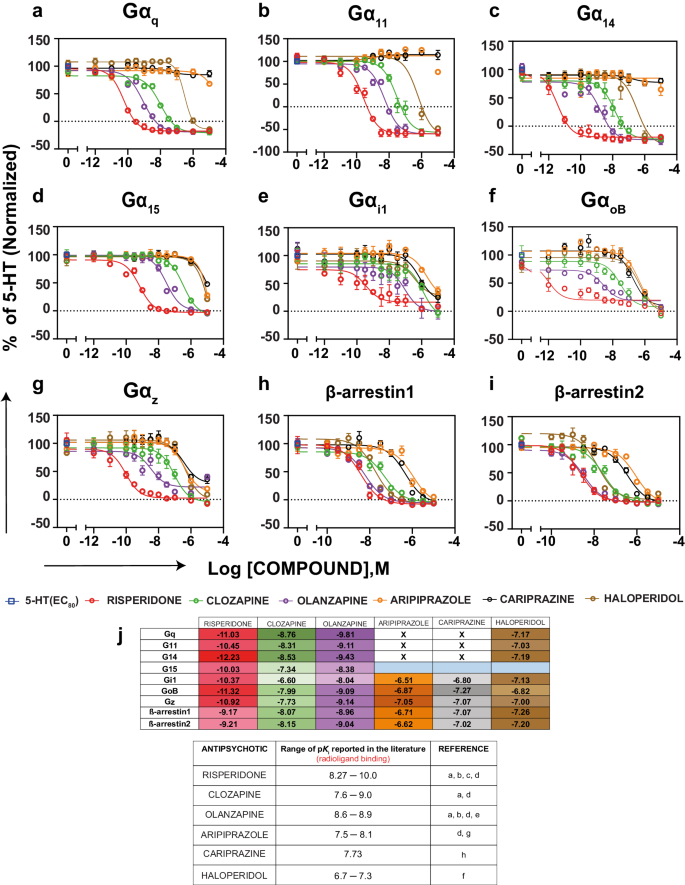
We examined the antipsychotics for his or her impact on the 5-HT (EC80)-mediated activation of the totally different G protein pathways and on the recruitment of β-arrestins. As was noticed within the agonist mode, the inverse agonist exercise of risperidone, clozapine, olanzapine and haloperidol may very well be seen at Gαq, Gα11 and Gα14 as mirrored by the truth that the compounds not solely absolutely antagonized the 5-HT-promoted BRET response but in addition promoted a BRET discount beneath basal exercise (Fig. 4a–c and Supplementary Desk 4b). For Gα15, Gαz, Gαi1, GαoB and the β-arrestins, the 4 compounds behave as full antagonists and no inverse agonist exercise may very well be detected (Fig. 4d-i and Supplementary Desk 4b).
a-i Focus response curves with the exercise of the six antipsychotics examined within the antagonist mode (i.e; within the presence of an EC80 focus of 5-HT) utilizing the ebBRET-based EMTA biosensors in HEK293 cells heterologously expressing the untagged 5-HT2AR and the biosensor (rGFP-CAAX together with p63-RlucII for Gαq household, Rap1GAP-RlucII for Gαi/o/z, and the respective Gα subunits, in addition to β-arrestin1-RlucII or β-arrestin2-RlucII and WT-GRK2 for recruitment of β-arrestins). Outcomes are expressed as % response of 5-HT (EC80) on the respective pathways (normalized with respect to the response of 5-HT (EC80)) (imply ± SEM; n = 3–5). j Heatmap depicting the equilibrium dissociation fixed (Okb) of the antipsychotics calculated primarily based on the modified Cheng-Prusoff equation as described within the strategies part together with the pOki values reported within the literature (a [66]; b [67]; c [68]; d [69]; e [70]; f [28]; g [56]; h [71]). The empty cells with a cross point out no exercise within the antagonist mode whereas cells coloured blue point out IC50 > 10 µM.
On account of being excessive efficacy partial agonists at Gαq, Gα11 and Gα14, aripiprazole and cariprazine didn’t present antagonistic exercise for the 5-HT (EC80)-mediated activation of those pathways (Fig. 3 and Supplementary Tables 3, 4a, b). In distinction, the 2 compounds have been low efficiency full antagonists for GαoB, Gαz and β-arrestins, and in lieu of their weak partial agonist exercise at Gα15 and Gαi1, they’re partial antagonists with comparatively excessive inhibitory efficacy at these pathways (inhibiting the 5-HT promoted responses by ~80% at Gαi1; Figs. 3, 4 and Supplementary Desk 4a, b). These information point out that aripiprazole and cariprazine have pathway selective efficacies on the 5-HT2AR.
Though not a direct indication of binding affinity, the IC50 values obtained on the totally different pathways have been used to calculate the obvious equilibrium dissociation constants (Okb) of the antipsychotics (Fig. 4j) primarily based on the modified Cheng-Prusoff equation [41]. Total, the obvious affinities calculated from the purposeful information observe the order of measured affinities reported within the literature. Nonetheless, the analyses reveal the existence of distinct functionally-derived obvious affinities for the totally different pathways thought of, indicating that affinities derived from binding experiments can’t be used on to predict how potent a drug will probably be at inhibiting a particular pathway. Good examples of this are offered by risperidone and olanzapine. Risperidone displayed a Okb 1000-times decrease to inhibit Gαq than to dam β-arrestin recruitment. For its half, the Okb of olanzapine to inhibit Gαq and inhibit the 5-HT-mediated β-arrestin recruitment have been related, however 100-fold decrease than for inhibiting Gαi1.
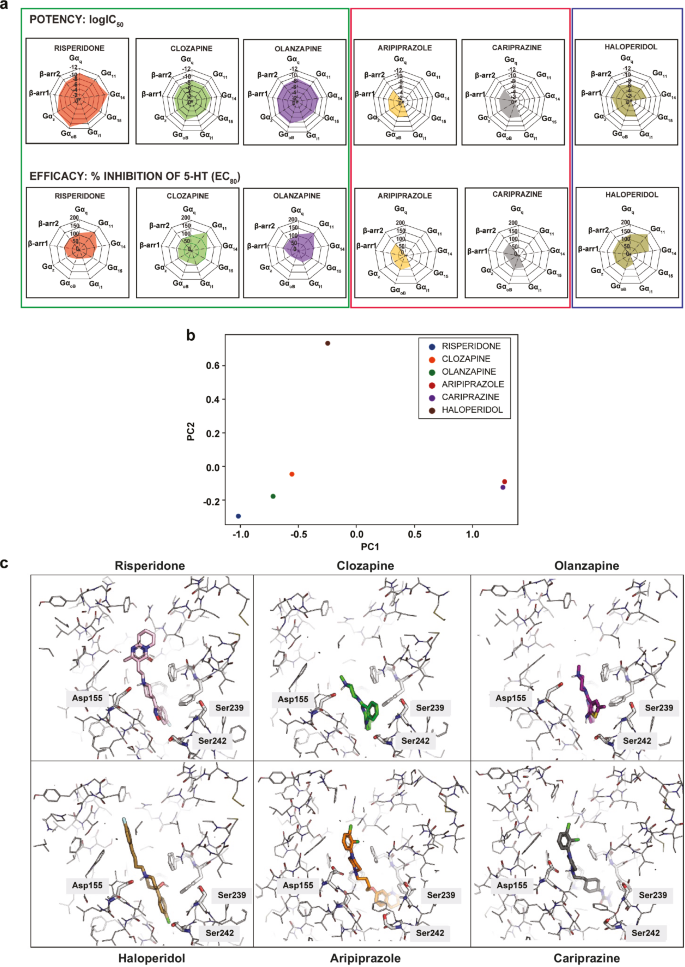
Determine 5 illustrates the general antagonist and inverse agonist transducer engagement profiles noticed for the six antipsychotic medication. The online illustration permits to obviously see that the medication could be labeled into three totally different clusters in line with their relative efficacies and potencies towards the totally different G protein subtypes and β-arrestins. Risperidone, clozapine and olanzapine displayed what may very well be thought of as a balanced profile since every of the medication confirmed related potencies and efficacies among the many pathways. In sharp distinction, aripiprazole and cariprazine confirmed marked pathway desire, having antagonistic exercise solely at a subset of the pathways. Haloperidol fell right into a class of its personal, the place pathway desire may very well be seen solely when contemplating its potencies for the totally different pathways. Of great discover, the three clusters correspond to the classification of those antipsychotics as outlined by their therapeutic profiles, specifically typical (haloperidol), atypical (risperidone, clozapine and olanzapine) and third technology (aripiprazole and cariprazine). This visible clustering primarily based on their general signaling profiles was additional confirmed utilizing principal part evaluation of the information (Fig. 5b).
a Net illustration of the efficiency (logIC50) and efficacy (% inhibition) of the six antipsychotics examined within the antagonist mode. b Principal part evaluation of the antagonist mode (efficiency and efficacy) depicting the hierarchical clustering of the antipsychotics into three distinct teams. c Binding modes of the six antipsychotics on the 5-HT2AR.
Given the clear separation into three teams on the pathway degree, we have been questioning whether or not this was additionally borne out on the structural degree. To that finish, we analyzed the present experimental structural information for risperidone (6A93) [50], aripiprazole (7VOE) [51], and cariprazine (7VOD) [51] and generated docking-derived poses for the remaining three molecules. As could be seen in Fig. 5c, the poses of olanzapine and clozapine overlay properly with the X-ray construction of the risperidone-bound receptor. Their three-ring (clozapine and olanzapine) and two-ring (risperidone) methods, respectively, are positioned deep within the pocket, near Ser239, a residue that has been described as being concerned in receptor activation networks [52]. The truth that within the poses of all three ligands hydrogen bond donors or acceptors are near the facet chain of Ser239 would possibly level to a job of this residue of their related pathway signatures (Fig. 5a). Aripiprazole and cariprazine are positioned even deeper alongside the z-axis, pointing into the house between helices 5 and 6. The binding modes of those two medication are considerably overlapping, and the residues surrounding the deepest-binding parts are once more made up of residues corresponding to Phe332 (6.44) and V333 (6.45) implicated in receptor activation [52, 53], probably resulting in a definite conformation which may very well be accountable for their partial agonism. We wish to be aware, nonetheless, that our docking calculations additionally yielded different poses for aripiprazole and cariprazine that sure extra within the course of the extracellular house. In these poses, the dihydroquinoline and urea motifs, respectively, are concerned in increased numbers of polar contacts in comparison with the experimental constructions (Supplementary Fig. 7). Nonetheless, even in these different poses, aripiprazole and cariprazine are extremely congruent, offering a attainable rationale for his or her related signaling profiles. Lastly, the pose of haloperidol is positioned considerably in-between the opposite two areas, and lacks the cumbersome rings near Ser239 that may be a widespread motif for clozapine, olanzapine and risperidone. These analyses help the notion that the binding modes additionally cluster in 3 totally different teams according to the signaling signature.