Syt11 deficiency is linked to schizophrenia
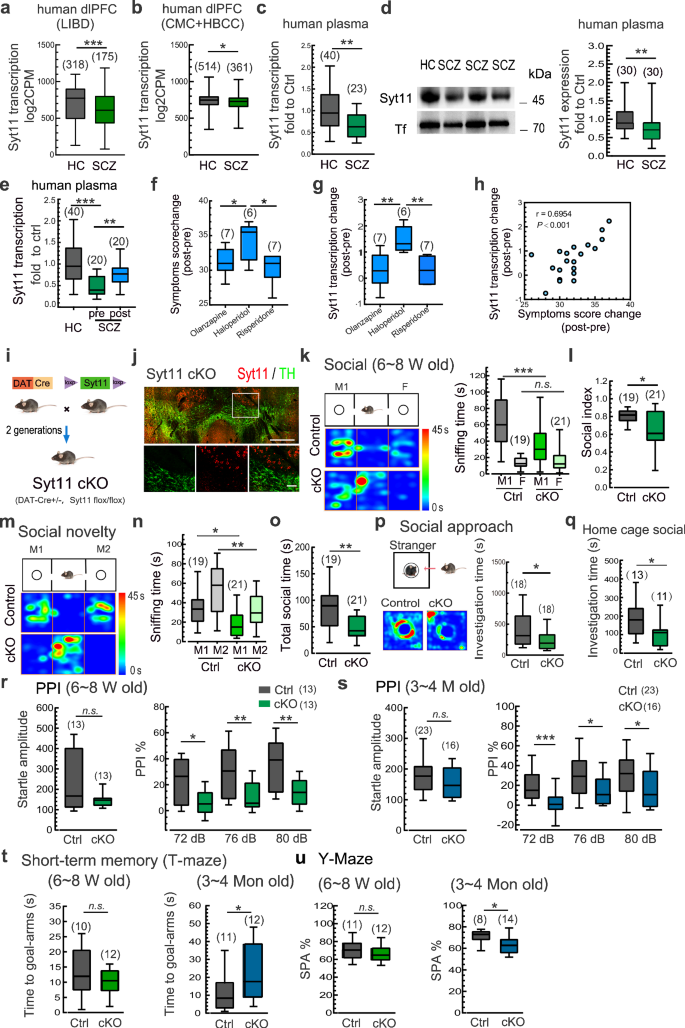
To investigate the potential association between Syt11 expression and schizophrenia, we analyzed Syt11 expression in three datasets of human brain tissues: the Lieber Institute for Brain Development (LIBD), the CommonMind Consortium (CMC), and the Human Brain Collection Core (HBCC). These datasets included mRNA transcription profiles from the dorsolateral prefrontal cortex (dlPFC) of postmortem brains from both schizophrenia patients and healthy controls. To minimize site-specific technical variations, the CMC and HBCC datasets, which underwent RNA extraction and data generation at a single facility, were combined31. Our analysis revealed a significant reduction in Syt11 expression in prefrontal cortex tissues of schizophrenia patients (Fig. 1a, b). This finding was further confirmed by qPCR and Western blot analyses, which showed a significant decrease in both Syt11 transcription and protein expression in the plasma of schizophrenia patients in two independent case-control samples (Fig.1c, d). Specifically, ~50% of schizophrenia patients showed a clear reduction of Syt11 expression in the plasma (by setting the threshold at the 90th percentile of the highest value in the healthy control group), with an area under the curve ratio (AUC) of 0.737 in the receiver operating characteristic (ROC) curve (Supplementary information, Fig. S1). These results not only identify Syt11 deficiency as a potential risk factor for the pathogenesis of schizophrenia, but also define plasma Syt11 as a biomarker for the clinical diagnosis of schizophrenia.
a, b Transcript expression levels of Syt11 in the dlPFC of postmortem brains from schizophrenia (SCZ) patients vs healthy controls (HC). RNA-sequencing data set were obtained from the Lieber Institute for Brain Development (LIBD), the CommonMind Consortium (CMC), and the Human Brain Collection Core (HBCC). The CMC and HBCC data sets were performed at a single facility with similar processes and thus combined to minimize site-specific sources of technical variation. c Transcript expression levels of Syt11 in peripheral blood from SCZ patients vs HC. d Representative western blots and expression levels of Syt11 in plasma from SCZ patients vs HC. e Transcript expression levels of Syt11 in peripheral blood from HC and SCZ patients before (SCZ-pre) and after (SCZ-post) antipsychotic treatment. f Changes in SCZ symptoms scores of SCZ patients after treatment with olanzapine, haloperidol, or risperidone. g Transcript expression changes of Syt11 in peripheral blood from SCZ patients after treatment with olanzapine, haloperidol, or risperidone. h Pearson correlation analysis between changes in Syt11 expression and changes in SCZ symptom scores after antipsychotic treatment as in (e–g). i Schematic of the generation of DA neuron-restricted Syt11 conditional knockout (cKO) mice. j Representative micrograph showing the immunostaining of Syt11 (red) and TH (green) in a VTA-containing slice (enlarged insets in the lower panel). Scale bars: 500 μm (upper), 100 μm (lower). Data from 3 mice. k, l Schematic, representative heat maps, and statistics of the three-chamber social interaction test of juvenile (6–8 weeks) Syt11-cKO or DAT-Cre (Ctrl) mice. M1, a novel mouse; F, fake toy mouse. Sniffing time and social index of Syt11-cKO vs control mice were used for analysis. m Schematic and representative heat maps of the three-chamber social novelty test of juvenile Syt11-cKO vs control mice. M1, familiar mouse (the former novel mouse in k and l); M2, new comer novel mouse. n, o Statistics of sniffing time (with M1 or M2) and total social time (sniffing with M1 and M2) of Syt11-cKO vs control mice. p Left, schematic and representative heat maps of the social approach test. Right, statistics of sniffing time with a caged novel mouse of Syt11-cKO vs control mice (6–8 weeks). q Statistics of sniffing time with an intruder mouse of Syt11-cKO vs control mice (6–8 weeks) in the home-cage social test. r, s Statistics of startle responses and pre-pulse inhibition (PPI) of juvenile (6–8 weeks) and adult (3-4 months) Syt11-cKO mice vs control mice. t Statistics of short-term memory (T-maze) of juvenile and adult Syt11-cKO mice vs control mice. u Statistics of the spontaneous alternation Y-maze test of juvenile and adult Syt11-cKO mice vs control mice. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Two-tailed Mann-Whitney test for (a-d, l, o-q, r-u), Pearson correlation analysis for (h), one-way ANOVA for (f, g), or Ordinary two-way ANOVA followed by Bonferroni’s multiple comparisons for (e, k, n), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
To validate the close association between Syt11 expression and schizophrenia, we next examined Syt11 expression in plasma samples collected from healthy individuals and schizophrenia patients before and after antipsychotic treatment. We found that the decreased expression of Syt11 in the plasma of schizophrenia patients was restored after the antipsychotic treatment with olanzapine, haloperidol, or risperidone (Fig. 1e). Specifically, patients who received the haloperidol treatment showed the most substantial changes in symptom scores when compared to those treated with olanzapine or risperidone, and this was correlated with the highest restoration of Syt11 expression in the haloperidol-treated patients (Fig. 1f, g). Importantly, Pearson’s correlation analysis demonstrated a positive correlation between the overall changes in schizophrenia symptom scores and the changes in Syt11 expression after antipsychotic treatment (Fig. 1h), suggesting that the rescue of Syt11 deficiency in schizophrenia patients is closely associated with the therapeutic effects of antipsychotic medications. Collectively, these findings from human samples and clinical treatment demonstrate the close association between Syt11 deficiency and schizophrenia.
DA neuron-restricted knockout of Syt11 leads to schizophrenia-like behaviors
To investigate the potential role of SYT11 deficiency in initiating the pathogenesis of schizophrenia, we generated DA neuron-restricted Syt11 cKO mice by crossing homozygous floxed Syt11-null mice with DAT-driven Cre recombinase (DAT-Cre) transgenic mice (Fig. 1i), as previously described30. Immunostaining for tyrosine hydroxylase (TH) confirmed the specific loss of Syt11 in midbrain DA neurons (Fig. 1j). The three-chamber social interaction test was employed to assess the social disability, a prominent negative symptom in schizophrenia, in young (6-8 weeks old) male Syt11-cKO mice (Fig. 1k). As expected, control mice (DAT-Cre) spent more time interacting with a stranger mouse (M1) than a fake mouse (F) (Fig. 1k). In contrast, Syt11-cKO mice showed reduced sniffing time with the M1 mouse, while their interaction with the F mouse remained unchanged, resulting in the decreased social preference index (Fig. 1k, l), suggesting impaired social preference upon Syt11 deficiency in DA neurons. Similarly, in the social novelty test, Syt11-cKO mice showed the reduced social preference for a new stranger mouse (M2) over a familiar one, in contrast to control mice (Fig. 1m, n). Importantly, the total sniffing time with both M1 and M2 mice decreased greatly in Syt11-cKO mice (Fig. 1o), confirming the impaired social activity in the absence of Syt11 in DA neurons.
Furthermore, we applied the social approach test to further evaluate the behavioral deficits in young Syt11-cKO mice (Fig. 1p). Compared to controls, Syt11-cKO mice spent significantly less time approaching and interacting with a caged stranger mouse (Fig. 1p). The home-cage social test also revealed reduced social interaction of Syt11-cKO mice with a stranger intruder mouse (Fig. 1q), confirming that the DA neuron-restricted KO of Syt11 is sufficient to induce social deficits at early ages.
To examine whether these social deficits persist into adulthood, we conducted the same social behavioral tests with adult (3 months old) male Syt11-cKO mice. These mice also showed reduced sniffing time with the M1 mouse and thus a decreased social preference index (Supplementary information, Fig. S2a-c). Similarly, cKO mice performed worse than controls in the social novelty test (Supplementary information, Fig. S2d-f). Similar social deficits were observed in Syt11 cKO mice at 1 year old (Supplementary information, Fig. S2i,j), indicating the long-lasting social withdrawal in the absence of Syt11. Additionally, adult cKO mice also showed decreased social interaction time with the stimulus mouse in the social approach test (Supplementary information, Fig. S2g). Consistent with this, the social interaction time of adult Syt11-cKO mice with the stranger intruder mouse was shorter than that of controls (Supplementary information, Fig. S2h). Together, these results suggest an early-onset and enduring social withdrawal phenotype in DA neuron-restricted Syt11-cKO mice.
To further confirm the association of social withdrawal in Syt11-cKO mice with schizophrenia, we carried out a series of behavior analyses to examine other schizophrenia-related symptoms. In the locomotion test, Syt11-cKO mice showed the increased total travel distance with intact travel speed at adolescence (Supplementary information, Fig. S2k, l), indicating locomotion hyperactivity, which corresponds to the psychomotor agitation observed in schizophrenia patients. Furthermore, both adolescent and adult Syt11-cKO mice exhibited aberrant prepulse inhibition (PPI) of the acoustic startle response (Fig. 1r, s), a well-defined hallmark of sensorimotor gating dysfunction manifested in early adulthood in patients with schizophrenia. As a control, the intact startle amplitude indicated normal gross auditory and motor ability in Syt11-cKO mice (Fig. 1r, s). Although Syt11-cKO mice did not show clear impairments in the T-maze test during adolescence, adult cKO mice spent more time turning into the goal arm (Fig. 1t), suggesting deficits in short-term working memory upon Syt11 deficiency in DA neurons. In addition, the adult Syt11-cKO mice also performed poorly in a spontaneous alternation Y-maze test (Fig. 1u), consistent with the cognitive dysfunction observed in patients with schizophrenia from early adulthood. In contrast, the cKO mice did not show enhanced marble-burying behavior (Supplementary information, Fig. S2m) or excessive self-grooming in the open field test (Supplementary information, Fig. S2n), suggesting the absence of repetitive behaviors associated with autism32. Overall, these results demonstrate a role of Syt11 deficiency in DA neurons in mediating the pathogenesis of schizophrenia and provide a mouse model for schizophrenia study, particularly that related to social withdrawal and other negative symptoms.
Syt11 deficiency in early adolescence mediates social deficits
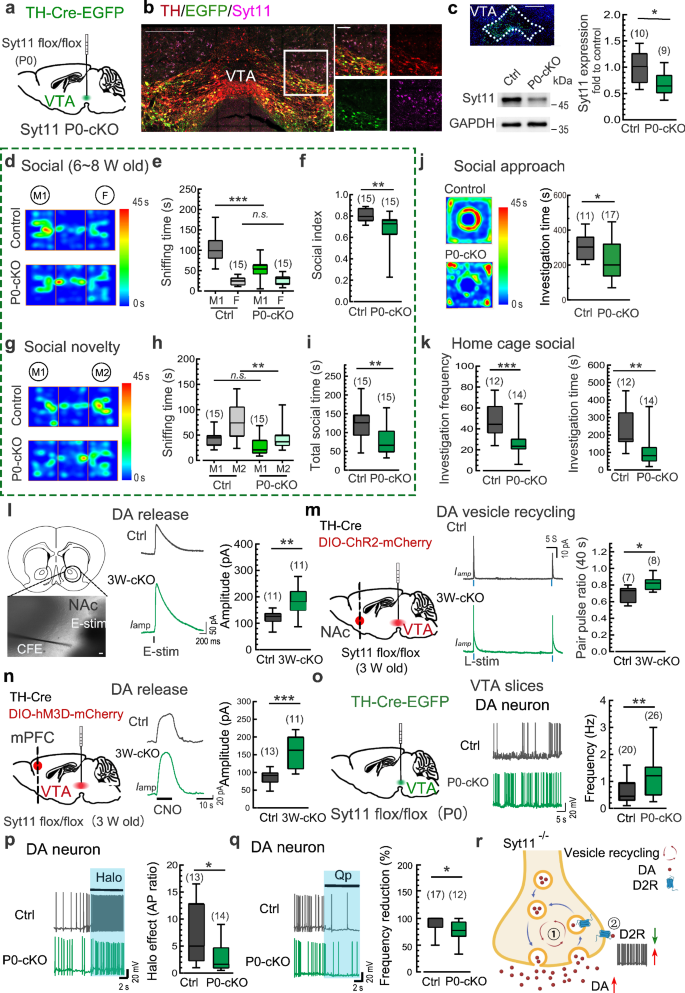
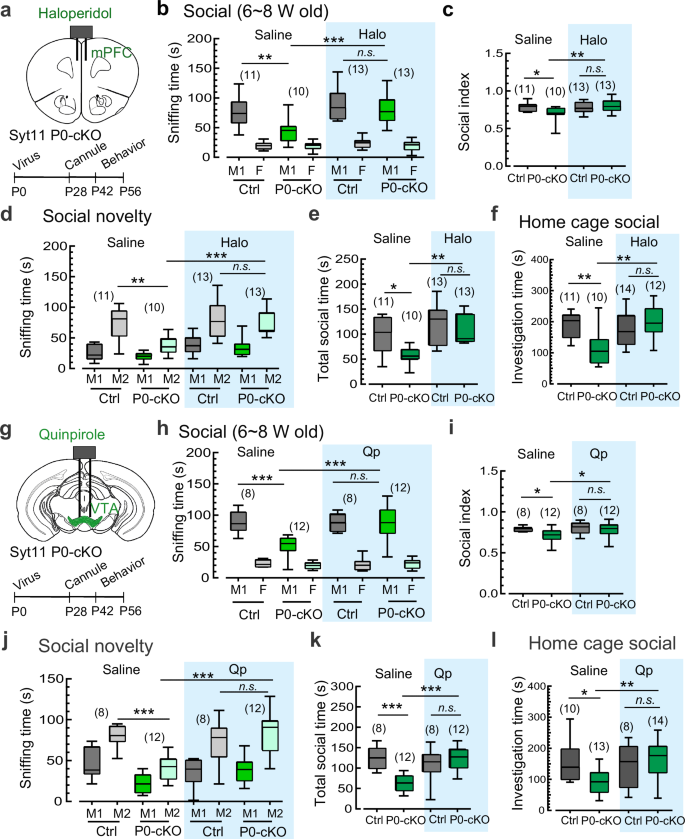
We aimed to investigate whether there is a sensitive time-window during which Syt11 deficiency leads to social deficits. To address this issue, we generated DA neuron-restricted knockout of Syt11 during early adolescence and adulthood, respectively. To generate DA neuron-restricted knockout of Syt11 at early adolescence, we stereotaxically injected a TH-Cre expressing AAV9 virus into the VTA of homozygous floxed Syt11-null mice on postnatal day 0–1 (P0, Fig. 2a). Immunostaining confirmed the absence of Syt11 in virus-infected DA neurons (GFP-positive) in the VTA 6 weeks after virus injection (Fig. 2b; Supplementary information, Fig. S3a, b), and Western blot analysis validated the decreased expression of Syt11 in the ventral midbrain (Fig. 2c). Notably, DA neuron-restricted KO of Syt11 since P0 (P0-cKO) exhibited decreased sniffing time with M1 mice and a reduced social index in the three-chamber social interaction test during 6–8 weeks of age (Fig. 2d-f). Similarly, these Syt11 P0-cKO mice also showed decreased social preference for the stranger M2 mouse and reduced total social time with both mice compared with controls (Fig. 2g-i). In addition, Syt11 P0-cKO mice showed reduced social interaction with a caged stranger mouse in the social approach test (Fig. 2j) and the stranger intruder mouse in the home-cage social test (Fig. 2k). These findings indicate that the absence of Syt11 at an early age can mediate impairments in social ability. Importantly, adult Syt11 P0-cKO mice also showed impaired social behaviors in the three-chamber social test, social novelty test, and social approach test (Supplementary information, Fig. S3), suggesting that Syt11 deficiency since P0 is associated with ongoing social deficits.
a Schematic representation of virus injection (TH-Cre-EGFP, or TH-EGFP served as a control) into the VTA of neonatal Syt11-flox/flox mice (P0) for the generation of DA neuron-restricted Syt11-cKO mice from birth (Syt11 P0-cKO). b Representative micrograph showing the immunostaining of Syt11 (magenta) and TH (red) in a VTA-containing slice from a Syt11 P0-cKO mouse (6 weeks post virus injection) as described in a. Enlarged insets are shown on the right. n = 3 mice; Scale bars: 400 μm (left), 50 μm (right). c Representative Western blots and statistics showing the expression of Syt11 in the VTA of Syt11 P0-cKO mice compared to control mice. Scale bars, 400 μm. d–f Representative heat maps and statistics of the three-chamber social interaction test of juvenile (6–8 weeks) Syt11 P0-cKO vs control mice. g–i Representative heat maps and statistics of the social novelty test of juvenile Syt11 P0-cKO vs control mice. j Representative heat maps and statistics of sniffing time in the social approach test of juvenile Syt11 P0-cKO vs control mice. k Statistics of investigation frequency and investigation time in the home-cage social test of juvenile Syt11 P0-cKO vs control mice. l Schematic, representative amperometric currents (Iamp), and statistics showing DA release from DAergic terminals in the NAc of Syt11 P0-cKO (n = 3) vs control (n = 3) mice. Scale bars, 10 μm. m Left, schematic showing the co-injection of TH-Cre and DIO-ChR2-mCherry viruses into the VTA of juvenile (3 weeks) Syt11-flox/flox or wide-type mice to generate Syt11 3W-cKO (n = 3) or control (n = 3) mice with ChR2 expressed in VTADA neurons. Middle and right, representative paired-pulse stimulus (40 s)-evoked amperometric signals and statistics of the paired-pulse ratio showing the recycling of DA vesicles in NAc slices from Syt11 3W-cKO vs control mice. n Schematic showing the co-injection of TH-Cre and DIO-hM3D-mCherry viruses into the VTA of juvenile (3 weeks) Syt11-flox/flox or wide-type mice to generate Syt11 3W-cKO (n = 3) or control (n = 3) mice with hM3D expressed in VTADA neurons. Middle and right, representative amperometric current (Iamp) traces and statistics of DA release in the mPFC of Syt11 3W-cKO vs control mice following CNO application (5 μM). o Schematic of virus injection (TH-Cre-EGFP or TH-EGFP) into the VTA of neonatal Syt11-flox/flox mice (P0) for the generation of Syt11 P0-cKO (n = 8) or control (n = 4) mice. Middle and right, representative AP traces and statistics of the spontaneous action potential firing rate of VTA DA neurons from Syt11 P0-cKO mice vs control mice. p Representative AP traces and statistics showing the excitatory effect of the D2R antagonist haloperidol (Halo, 50 nM) on DA neurons in VTA slices in situ from Syt11 P0-cKO (n = 4) vs control (n = 4) mice. q Representative AP traces and statistics showing the inhibitory effect of the D2R agonist quinpirole (Qp, 50 nM) on the excitability of DA neurons in VTA slices in situ from Syt11 P0-cKO (n = 5) vs control (n = 5) mice. r A working model showing that Syt11 deficiency increases DA transmission via ① facilitating DA vesicle recycling, and ② decreasing surface auto-receptor D2R expression, which leads to increased excitability of DA neurons. Created in BioRender. Yang, C. (2023) https://BioRender.com/w74v548. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s multiple comparisons for (e, h) or two-tailed Mann-Whitney test for (c, f, i–q), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
To test whether Syt11 knockout in adult mice also results in social deficits, we generated Syt11 adult-cKO mice by injecting TH-Cre virus into the VTA of adult (3-4 months) Syt11-flox mice (Supplementary information, Figs. S4). Although Syt11-cKO led to increased DA release in the nucleus accumbens (NAc, Supplementary information, Fig. S5a) as revealed by electrochemical amperometric recordings, these mice spent similar amounts of time interacting with the M1 mouse and thus showed an unchanged social index in the three-chamber social interaction test (Supplementary information, Fig. S5b-d). In addition, the social preference for the M2 mouse and the total social time with both mice remained intact in adult-cKO mice compared with controls (Supplementary information, Fig. S5e-g). Syt11 adult-cKO mice also showed similar social interaction time with the caged stranger mouse in the social approach test (Supplementary information, Fig. S5h) and the stranger intruder mouse in the home-cage social test (Supplementary information, Fig. S5i). Collectively, these results demonstrate that Syt11 adult-cKO mice exhibit normal social behaviors, suggesting that there is a sensitive time-window before adult for Syt11 deficiency-mediated schizophrenia-like social deficits, further supporting the persistence of social deficits when Syt11 deficiency occurs at earlier ages, and implying a potential role of Syt11 in neural development.
Syt11 deficiency leads to DA over-transmission via the accelerated vesicle recycling and off-membrane trafficking of D2Rs
Our previous studies have shown that Syt11 serves as a clamp for endocytosis and thus inhibits vesicle replenishment and DA release, while Syt11 cKO in midbrain DA neurons leads to excessive DA release in the striatum27,29,30. Here, we further investigated whether Syt11 cKO in VTADA neurons leads to social deficits via DA over-transmission. To examine this, we applied amperometric recordings with electrochemical carbon fiber electrodes (CFEs) in the NAc and the medial prefrontal cortex (mPFC), which are DA neuron-projecting regions involved in social behaviors. Consistent with previous reports30,33, local electrical pulse-stimulation induced a transient increase in amperometric current (Iamp), followed by a subsequent decay to the baseline, representing transient DA release in the NAc (Fig. 2l). As expected, DA neuron-restricted KO of Syt11 in the VTA led to increased DA release in the NAc (Fig. 2l). To specifically assess DA vesicle recycling in the NAc, we employed an optogenetic approach by injecting FLExloxP-based Channelrhodopsin-2 (ChR2)-expressing AAV9 virus and TH-Cre virus into the VTA of Syt11-floxed null or control mice. Notably, the paired-pulse ratio of DA release in NAc slices evoked by the 488-nm laser stimulus increased substantially in Syt11-cKO mice compared with control mice (Fig. 2m), validating accelerated vesicle recycling and hence elevated DA release in the NAc in the absence of Syt11.
We next assessed DA release in the mPFC by utilizing a clozapine-N-oxide (CNO)-based chemogenetic approach. TH-Cre and Cre-dependent hM3Dq-expressing AAV2/9 viruses were stereotaxically injected into the VTA of Syt11-floxed null or control mice (Fig. 2n). Immunostaining confirmed that the expression of mCherry/hM3Dq was restricted to DA neurons in the VTA (Supplementary information, Fig. S6a, b). Patch-clamp recordings revealed an elevated firing rate of DA neurons in the VTA upon CNO application (Supplementary information, Fig. S6c), while CFE recordings demonstrated that CNO application further elicited DA release in the mPFC (Fig. 2n). Similar with that in the NAc (Fig. 2l), the CNO-evoked DA release in the mPFC was higher in Syt11-cKO mice than in control mice (Fig. 2n). These results validate the elevated DA transmission and accelerated DA vesicle recycling upon Syt11 deficiency in DA neurons.
Moreover, electrophysiological patch-clamp recordings revealed a pronounced increase in the firing rate of action potentials (APs) of DA neurons in VTA slices from Syt11 P0-cKO mice (Fig. 2o), indicating the over-excitation of midbrain DA neurons in the absence of Syt11. Consistent with this, we also found the increased resting membrane potential (RMP) in VTADA neurons, with the membrane capacitance (Cm) and input membrane resistance (Rm) remained unchanged (Supplementary information, Fig. S7a). We hypothesized that the surface auto-inhibitory D2R receptor in DA neurons may undergo alterations due to the accelerated vesicle recycling27,28,30,34. Consistent with our expectation, the facilitatory effect of the D2R antagonist haloperidol on AP firing of DA neurons was substantially diminished in VTA slices from Syt11 P0-cKO mice (Fig. 2p; Supplementary information, Fig. S7b). Similarly, we also observed a decreased inhibitory effect of the D2R agonist quinpirole on AP firing (Fig. 2q; Supplementary information, Fig. S7b), suggesting a reduction in functional D2R in DA neurons in situ in the absence of Syt11. In line with this, we also observed accelerated endocytosis with FM uptake (Supplementary information, Fig. S7c) and decreased expression of membrane D2R (Supplementary information, Fig. S7d, e) in Syt11 knockdown dopaminergic SY5Y cells, as well as the decreased total D2R expression in the VTA in Syt11-cKO mice (Supplementary information, Fig. S7f). Although we couldn’t fully exclude other possibilities (i.e. receptor signaling adaptations, gene expression alterations), our recent reports27,29,30 and the present findings collectively underscore that Syt11 deficiency leads to elevated DA transmission via accelerated endocytosis and vesicle recycling, as well as the hyperactivity of DA neurons due to the increased off-membrane trafficking of surface D2Rs (Fig. 2r).
DA neuron over-excitation during adolescence mediates schizophrenia-related social deficits
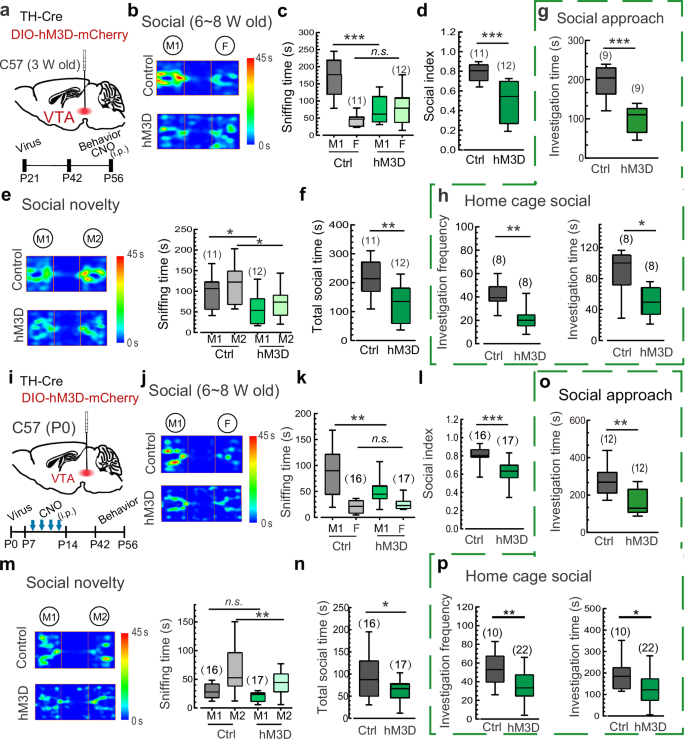
To further determine whether the over-excitation of VTADA neurons, which mimics elevated DA release, is sufficient to mediate schizophrenia-like social withdrawal, we performed co-injections of TH-Cre virus and Cre-dependent hM3Dq-expressing virus (with DIO-mCherry blank virus as a control) into the VTA of 3-week-old mice. These mice were then subjected to social behavior tests during adolescence (6–8 weeks) following the chemogenetic activation of DA neurons via intraperitoneal (i.p.) administration of CNO (Fig. 3a). Notably, a single dose of CNO was capable of significantly reducing the time spent sniffing the M1 mouse in the three-chamber social test by hM3Dq-expressing mice (Fig. 3b, c). The social preference index was also decreased greatly compared to control virus-injected mice (Fig. 3d). Similarly, chemogenetic activation of VTADA neurons also resulted in inferior performance of hM3Dq-expressing mice in the social novelty test (Fig. 3e, f). Furthermore, the social interaction time with the stimulus mouse in the social approach test and with the stranger intruder mouse in the home-cage social test were both reduced in mice following chemogenetic activation (Fig. 3g, h), confirming that hyperactivity of DA neurons during early development is sufficient to cause social deficits. Importantly, mice with chemogenetic activation also exhibited aberrant PPI in the acoustic startle response and impaired short-term memory (Supplementary information, Fig. S8), further supporting the association between DA over-transmission and social withdrawal in schizophrenia. In contrast, similar chemogenetic activation of DA neurons failed to induce social deficits in adult mice in the three-chamber social interaction test (Supplementary information, Fig. S9a-c), social novelty test (Supplementary information, Fig. S9d, e), and home-cage social test (Supplementary information, Fig. S9f). These results demonstrate that transient over-excitation of VTADA neurons only mediates social withdrawal before late adolescence or young adulthood, suggesting a critical time window for DA hyperactivity in the pathogenesis of schizophrenia and implying a development-dependent shift of DAergic circuit/pathway in social behaviors.
a Schematic showing the co-injection of TH-Cre and DIO-hM3D-mCherry/DIO-mCherry viruses into the VTA of juvenile (3 weeks) C57 mice. b–d Representative heat maps and statistics of the three-chamber social interaction test of juvenile (6–8 weeks) hM3D-expressing mice vs control mice following i.p. administration of CNO (0.5 mg/kg). e, f Representative heat maps and statistics of the social novelty test of juvenile hM3D-expressing mice vs control mice as described in b–d. g Statistics of sniffing time in the social approach test of juvenile hM3D-expressing mice vs control mice following i.p. administration of CNO. h Statistics of investigation frequency and investigation time in the home-cage social test of juvenile hM3D-expressing mice vs control mice following i.p. administration of CNO. i Schematic showing the co-injection of TH-Cre and DIO-hM3D-mCherry/DIO-mCherry viruses into the VTA of neonatal C57 mice (hM3D, P0) and the experimental procedure. j–l Representative heat maps and statistics of sniffing time and social index in the three-chamber social interaction test of juvenile repetitive CNO-treated (every second day during P7-P14) hM3D-expressing mice vs control mice as described in i. m, n Representative heat maps and statistics of sniffing time and total social time in the social novelty test of juvenile repetitive CNO-treated hM3D-expressing mice vs control mice. o Sniffing time of juvenile repetitive CNO-treated hM3Dq-expressing mice vs control mice in the social approach test with a caged novel mouse. p Investigation frequency and investigation time of juvenile repetitive CNO-treated hM3Dq-expressing mice vs control mice in the home-cage social test. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s multiple comparisons for (c, e, k, m), two-tailed Mann-Whitney test for (d, f–h, l, n–p), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
We further explored whether over-excitation of DA neurons before early adolescence leads to long-lasting social deficits resembling those observed in individuals with schizophrenia. To this end, we performed co-injections of TH-Cre virus and Cre-dependent hM3Dq-expressing virus into the VTA during the early postnatal period (P0). Subsequently, systemic administration of CNO (i.p.) every second day from P7 to P14 was delivered to induce sustained over-excitation of DA neurons during the synapse maturation phase (Fig. 3i). Notably, compared with control mice, hM3Dq-expressing mice showed a significant reduction in the time spent sniffing the M1 mouse, consistent with the decreased social preference index in the three-chamber social test 4–6 weeks after the CNO treatment (Fig. 3j-l). Furthermore, these CNO-treated hM3Dq-expressing mice also displayed the reduced social preference for the stranger mouse (M2) and consequently the reduced total social time with both mice in the social novelty test (Fig. 3m, n). In line with these results, CNO-treated hM3Dq-expressing mice exhibited pronounced impairments in social interactions with the stimulus mouse in the social approach test and the stranger intruder mouse in the home-cage social test (Fig. 3o, p). These findings indicate that the social deficits induced by repetitive chemogenetic activation of VTADA neurons during the P7–P14 period can persist until at least young adulthood. Interestingly, the over-excitation of VTADA neurons was maintained at least 4 weeks following the repetitive chemogenetic activation (Supplementary information, Fig. S10), suggesting a long-lasting plastic change in the excitability of DA neurons. These findings suggest that environmental disturbances leading to DA over-transmission at an early age are sufficient to initiate persistent schizophrenia-like behaviors.
Schizophrenia has been reported to be more prevalent and severe in men than in women2,13. Therefore, we investigated whether the over-excitation of DA neurons could similarly induce schizophrenia-like social withdrawal in female mice. Interestingly, repetitive chemogenetic activation of DA neurons before early adolescence resulted in persistent social deficits in female mice, ranging from late adolescence (Supplementary information, Fig. S11) to adulthood (Supplementary information, Fig. S12). These findings contradict the speculative ‘dual dysregulation’ of DA hypothesis15 and instead demonstrate that DA over-transmission is indeed a mechanism underlying social withdrawal. Considering the comprehensive schizophrenia-like behavioral changes observed in Syt11-cKO and Syt11 P0-cKO mice (Figs. 1 and 2; Supplementary information, Figs. S1-S5) and mice with DA neuron over-excitation (Figs. 2 and 3; Supplementary information, Figs. S6-S12), as well as the involvement of aberrant striatal DA release in the positive symptoms2, these findings suggest a scenario in which DA over-transmission may represent a shared pathway contributing to different symptoms of schizophrenia during a critical time window before late adolescence.
DA over-transmission in the mPFC during adolescence mediates social deficits
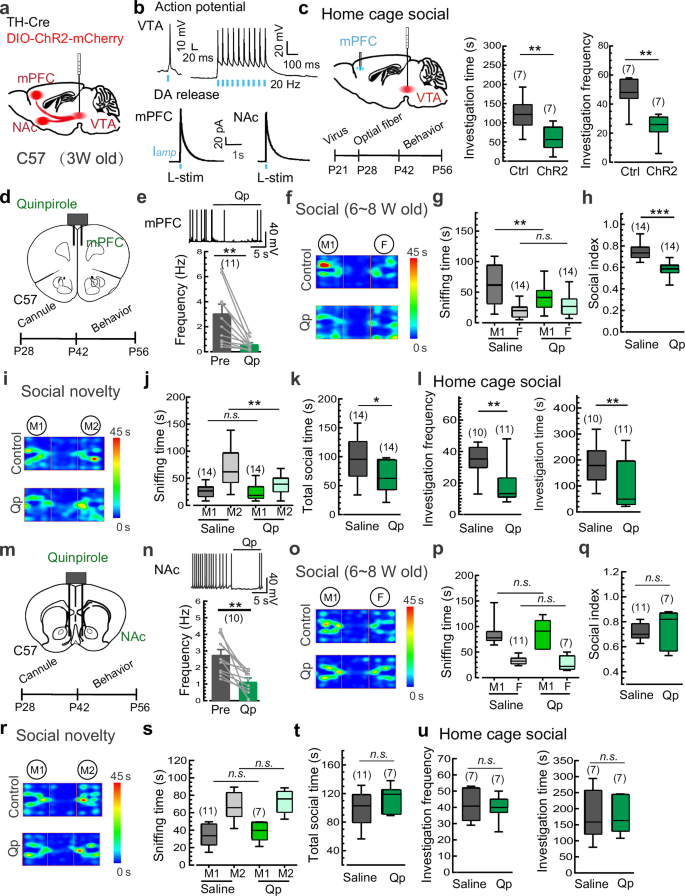
Given that the NAc and the mPFC are primary DA neuron-projecting regions involved in social behaviors, we used optogenetic manipulation to further determine the specific brain region downstream of VTADA neurons responsible for the social deficits in schizophrenia. The FLExloxP-based ChR2-expressing AAV9 virus and TH-Cre virus were co-injected into the VTA of 3-week-old mice (Fig. 4a). As expected, transient 473-nm light stimulation (L-stim, 1 ms duration) reliably elicited AP firing in DA neurons (mCherry-positive) in current-clamp electrophysiological recordings (Fig. 4b). Meanwhile, L-stim also triggered DA release in the NAc and mPFC, as detected by electrochemical amperometric recordings (Fig. 4b). Home-cage social test was carried out during the P42–P56 time window to evaluate the possible contribution of the mPFC and NAc to the social deficits. Notably, a train of burst L-stim (5 ms, 8 pulses at 30 Hz; once every 5 s) on DA terminals in the mPFC resulted in a pronounced reduction in the social interaction with the intruder mouse (Fig. 4c). In contrast to the facilitatory effect of VTA-NAc DA signals on social behaviors in adult mice35 (Supplementary information, Fig. S13), similar L-stim in the NAc failed to induce detectable changes in social interaction in adolescent mice (Supplementary information, Fig. S14), suggesting that the elevated DA release in the mPFC, but not the NAc, during adolescence mediates the social withdrawal downstream of VTADA neurons.
a Schematic of the co-injection of TH-Cre and DIO-ChR2-mCherry viruses into the VTA of juvenile C57 mice (3 weeks). b Upper, representative AP traces showing optogenetic activation of DA neurons in VTA slices (mCherry-positive) by 473-nm light stimulation (L-stim). Lower, representative amperometric traces showing the L-stim induced DA release in the NAc and mPFC slices. Data from 3 mice. c Schematic and statistics of sniffing time/frequency in the home-cage social test of the ChR2-expressing mice vs control mice following L-stim trains in the mPFC. d Schematic of bilateral cannula application of the D2R agonist quinpirole (Qp) into the mPFC and the experimental procedure. e Representative AP traces and statistics showing the inhibitory effect of Qp (50 nM) on the excitability of D2R-positive cortical neurons in the mPFC (Data from 3 mice and presented as mean ± SEM). f–h Representative heat maps and statistics of the three-chamber social interaction test of juvenile (6–8 weeks) mice following the local application of Qp vs saline in the mPFC. i–k Representative heat maps and statistics of the social novelty test of the Qp- vs saline-treated juvenile mice as described in f–h. l Statistics of investigation frequency and investigation time in the home cage social test of the Qp- vs saline-treated juvenile mice. m Schematic of bilateral cannula application of Qp into the NAc and the experimental procedure. n Representative AP traces and statistics showing the inhibitory effect of Qp (50 nM) on the excitability of D2R-positive cortical neurons in the NAc (Data from 3 mice and presented as mean ± SEM). o–q Representative heat maps and statistics of the three-chamber social interaction test of juvenile (6–8 weeks) mice following the local application of Qp vs saline in the NAc. r–t Representative heat maps and statistics of the three-chamber social novelty test of juvenile mice following the local application of Qp vs saline in the NAc. u Investigation frequency and investigation time in the home cage social test of juvenile mice following the local application of Qp vs saline in the NAc. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s post-hoc test for (g, j, p, s), paired two-tailed Student’s t-test for (e, n), or two-tailed Mann-Whitney test for (c, h, k, l, q, t, u), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
To further validate potential roles of DA over-transmission in the mPFC in social withdrawal, we assessed social deficits with pharmacological intervention targeting postsynaptic D2Rs in the mPFC (Fig. 4d). As expected36, the local application of quinpirole, a potent D2R agonist, effectively reduced the activity of D2R-positive cortical neurons in mPFC slices (Fig. 4e). Subsequently, we conducted social behavior tests on adolescent mice following the stereotaxic injection of quinpirole (bilateral, 1 µg/µl, 0.2 µl per side) into the mPFC to augment DA transmission by activating postsynaptic D2Rs (Fig. 4d). As expected, quinpirole decreased the time spent in social interaction with the M1 mouse and the social preference index in the three-chamber social test (Fig. 4f–h). Similar inhibitory effects of quinpirole were observed in the social novelty test (Fig. 4i–k) and the home-cage social test (Fig. 4l). Conversely, the similar local infusion of quinpirole into the NAc (Fig. 4m, n) failed to induce impairments in social activities in the three-chamber social test (Fig. 4o–q), the social novelty test (Fig. 4r–t), and the home-cage social test (Fig. 4u). These findings confirm that DA over-transmission in the mPFC, but not the NAc, during adolescence plays a central role in mediating schizophrenia-like social deficits. The time window-specific inhibition of social preference by DA over-transmission in the mPFC suggests a role of DA transmission in the development and connectivity of mPFC neurons before late adolescence.
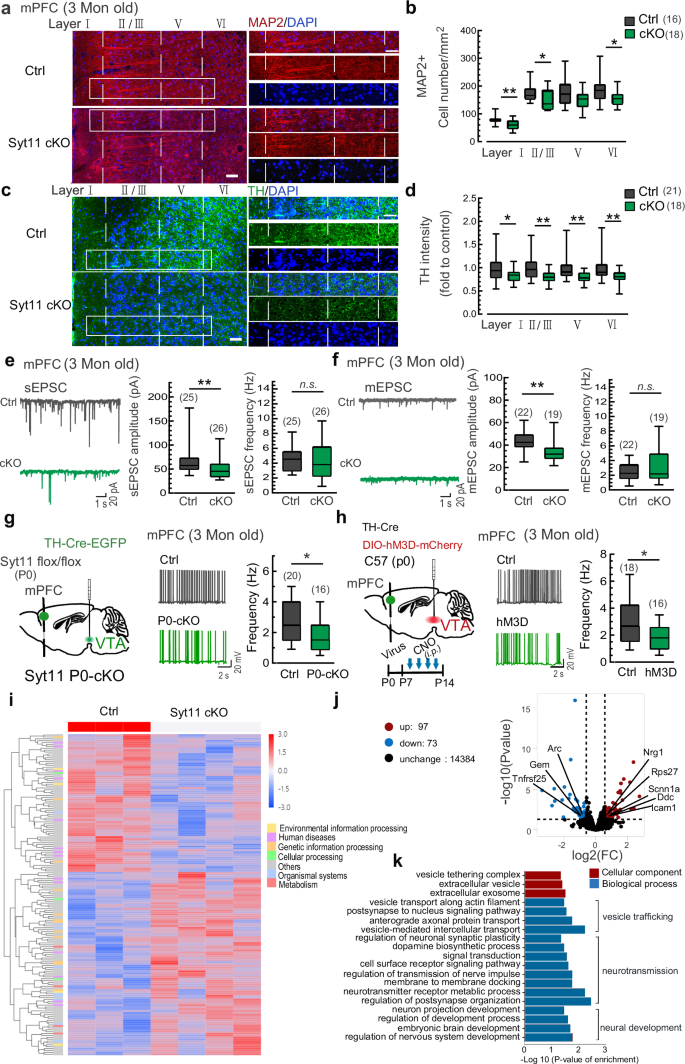
To validate potential roles of DA transmission in the plastic changes of mPFC neurons, we conducted assessments of morphological changes in the mPFC of Syt11 cKO mice. Consistent with the reduced cortical neurons and spine density observed in clinical studies37,38,39 and post-mortem evidence40,41,42, we observed a significant decrease of MAP2-positive neurons in Layers I, II/III, and VI of the mPFC from Syt11 cKO mice (Fig. 5a, b). Accordingly, the intensity of TH-positive neurites in all Layers I-VI was substantially reduced (Fig. 5c, d). Consistent with this, although the density of VTADA neurons in Syt11 cKO mice remained unchanged (Supplementary information, Fig. S15a,b), the dendritic complexity of these neurons decreased greatly (Supplementary information, Fig. S15c,d). In line with these findings, repetitive chemogenetic activation of VTADA neurons during P7-P14 induced similar long-term changes in MAP2-positive neurons and TH-positive neurites in the mPFC (Supplementary information, Fig. S16a–d). These results suggest that DA over-transmission in the mPFC at early ages is sufficient to induce neurostructual alterations in the mPFC. Subsequently, we performed patch-clamp electrophysiological recordings to assess the functional changes of mPFC cortical neurons. Consistent with the reduced cortical excitability observed in schizophrenia43, we indeed found the decreased excitatory postsynaptic current (Fig. 5e, f) and the decreased AP firing rate of mPFC cortical neurons in both adult Syt11 P0-cKO and CNO-treated (P7-P14) hM3Dq-expressing mice (Fig. 5g, h). These findings suggest that DA over-transmission before early adolescence leads to long-lasting morphological and functional plastic changes in mPFC cortical neurons.
a, b Representative micrographs and statistics of MAP2-positive neurons in the mPFC of adult Syt11-cKO (n = 6) vs control (n = 5) mice. Scale bars: 50 μm for left, 20 μm for right. c, d Representative micrographs and statistics of TH-positive neurites in the mPFC of adult Syt11-cKO (n = 6) vs control (n = 5) mice. e Representative sEPSC traces and statistics of the amplitude and frequency of sEPSC in mPFC cortical neurons from adult Syt11-cKO (n = 6) vs control (n = 6) mice. f Representative mEPSC traces and statistics of the amplitude and frequency of mEPSC in mPFC cortical neurons of adult Syt11-cKO (n = 6) vs control (n = 6) mice. g Left, schematic of virus injection (TH-Cre-EGFP/TH-EGFP) into the VTA of neonatal Syt11-flox/flox mice (P0) for the generation of Syt11 P0-cKO or control mice. Middle and right, representative AP traces and statistics of spontaneous AP firing rates in mPFC cortical neurons of adult (3 months) Syt11 P0-cKO (n = 4) vs control (n = 3) mice. h Left, schematic showing the co-injection of TH-Cre and DIO-hM3Dq-mCherry/DIO-mCherry viruses into the VTA of neonatal C57 mice (hM3D, P0) and the experimental procedure. Middle and right, representative AP traces and statistics of spontaneous AP frequency in mPFC cortical neurons of adult repetitive CNO-treated hM3Dq-expressing (n = 5) vs control (n = 4) mice. i The heatmap showing gene expression profiling determined by genome-wide RNA sequencing (RNA-Seq) of the mPFC in Syt11 cKO (n = 4) vs control (n = 3) mice. Rows represent differentially expressed genes (DEGs), and columns represent transcriptomic profiles of individual animals. j Volcano plots showing gene expression profiling of the mPFC in Syt11 cKO vs control mice. The x-axis represents log2 fold change (FC) between the two groups. k Ingenuity gene ontology (GO) analysis indicating significantly enriched GO terms in cellular components and biological processes. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Two-tailed Mann-Whitney test, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
To gain insights into mechanisms underlying the schizophrenia-like behavioral changes observed in Syt11 cKO mice, we performed genome-wide RNA-sequencing analysis to capture transcriptome-wide alterations in the mPFC of adult (3 months) Syt11 cKO mice. By comparing the gene expression profile of control (DAT-Cre) mice, we identified 170 differentially expressed genes (DEGs) in Syt11-cKO mice (Fig. 5i, j). Among these DEGs, 97 were upregulated and 73 were down-regulated. Interestingly, some of the detected DEGs, such as Nrg1, Arc, Ddc and Icam, are well-characterized schizophrenia risk genes that play critical roles in neural development and/or are functionally involved in the pathogenesis of schizophrenia44,45,46,47. Gene ontology (GO) analysis revealed that DEGs were enriched in cellular components involved in vesicle trafficking, such as the vesicle tethering complex, extracellular vesicles, and extracellular exosomes (Fig. 5k). In the biological process category, the enriched GO terms were strongly suggestive for processes related to vesicular trafficking (e.g. vesicle transport, axonal protein transport, post-synaptic retrograde transport, and vesicle-mediated intercellular transport), neurotransmission (e.g. DA biosynthetic process, membrane docking, synaptic transmission, synaptic plasticity, signal transduction, neurotransmitter receptor metabolic process, receptor signaling pathway, and post-synapse organization), and neural development (e.g. neural projection development, embryonic brain development, and nervous system development) (Fig. 5k). Consistently, repetitive chemogenetic activation of VTADA neurons during P7-P14 induced similar long-term transcriptome-wide changes in the mPFC (Supplementary information, Fig. S16e-g). Overall, the transcriptome sequencing analysis provides genetic insights into the long-term changes in the mPFC that are linked with Syt11 deficiency and/or DA over-transmission.
Pathogenic effects of the D2R antagonist haloperidol on social deficits
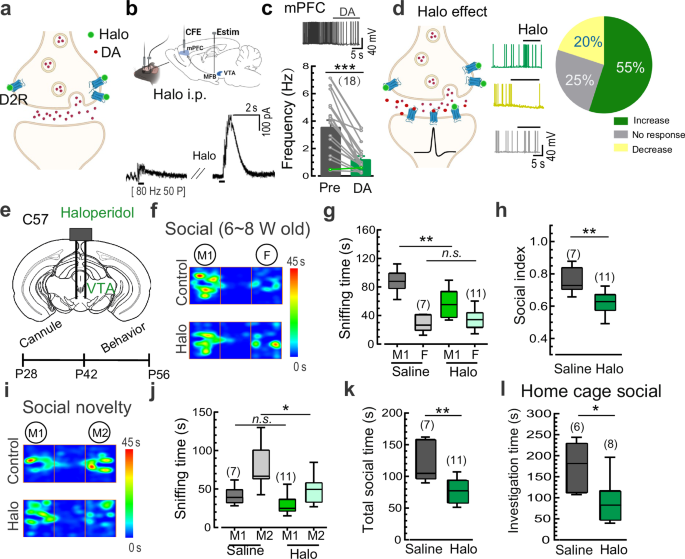
Haloperidol primarily alleviates positive symptoms by antagonizing D2 receptors and reducing elevated DA transmission in the striatum48, but its efficacy is limited and is often accompanied by adverse effects, including the worsening of negative symptoms5. Given that D2Rs also act as inhibitory auto-receptors in DA neurons49, we hypothesized that in addition to its therapeutic effect by antagonizing postsynaptic D2Rs, haloperidol may have an additional pathogenic effect on negative symptoms by removing the auto-inhibition of presynaptic/somatic D2Rs in DA neurons (Fig. 6a). Our patch-clamp recordings indeed showed that haloperidol application greatly increased the firing rate of DA neurons in the VTA (Supplementary information, Fig. S17). To further confirm the impact of haloperidol on DA release, we conducted electrochemical CFE recordings in the mPFC in vivo following electric stimulation of DA axons in the medial forebrain bundle. Notably, systemic administration of haloperidol (0.4 mg/kg) substantially enhanced evoked DA release in the mPFC (Fig. 6b), confirming the drug’s ability to induce aberrant DA release in vivo. Thereby, the increased DA levels led to a decrease in the firing rate of cortical neurons in the mPFC (Fig. 6c), exhibiting an opposite effect on cortical neuron excitability compared to the direct antagonism of postsynaptic D2Rs. Consistent with these findings, local application of haloperidol onto mPFC slices resulted in both increased (55%, post-synaptic effect) and decreased (20%, pre-synaptic effect) excitation of cortical neurons (Fig. 6d), indicating a mixed effect of haloperidol in the mPFC with the postsynaptic effect being predominant.
a Illustration showing the facilitatory effect of the D2R antagonist haloperidol (Halo) on DA transmission by targeting presynaptic D2Rs. b Schematic and representative traces showing increased DA release (evoked by a burst of electric stimulation [Estim, 50 pulses at 80 Hz] at the medial forebrain bundle) in the mPFC in vivo following i.p. application of Halo (0.4 mg/kg) in juvenile C57 mice. Data from 3 mice. c Representative trace and statistics of spontaneous APs of cortical neurons in mPFC slices in response to DA application (Data are presented as mean ± SEM). Data from 4 mice. d Left, illustration showing the dual effects of Halo on DA transmission by targeting presynaptic and postsynaptic D2Rs. Middle and right, representative traces and statistics of spontaneous APs of mPFC cortical neurons in response to Halo application. Data from 3 mice. e Schematic of bilateral cannula application of Halo into the VTA and the experimental procedure. f-h Representative heat maps and statistics of three-chamber social interaction time in juvenile (6–8 weeks) C57 mice following local application of Halo vs saline control in the VTA. i-k Representative heat maps and statistics of the social novelty test in Halo- vs saline-treated juvenile C57 mice. l Statistics of investigation time in juvenile C57 mice following local application of Halo vs saline control in the VTA in the home-cage social test. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s post-hoc test for (g, j), two-tailed paired Student’s t-test for (c), or two-tailed Mann-Whitney test for (h, k, l), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
Regarding opposing roles of presynaptic and postsynaptic D2Rs in DA transmission, we postulated that the lack of efficacy of haloperidol and other antipsychotic agents in treating negative symptoms of schizophrenia could be attributed to their dual disinhibitory effects on both presynaptic/somatic and postsynaptic D2Rs. To investigate this, we tested whether the modulation of presynaptic/somatic D2Rs by locally delivering the clinical drug haloperidol into the VTA (thereby removing the auto-inhibition of DA neurons) could induce schizophrenia-like social withdrawal in adolescent mice (Fig. 6e). Consistent with chemogenetic manipulations, pharmacological activation of DA neurons with a single local-delivery of haloperidol into the VTA (bilateral, 50 µM) resulted in impaired social behaviors, including reduced social interaction time with the M1 mouse and a decreased social preference index in the three-chamber social test (Fig. 6f-h). Similarly, haloperidol infusion into the VTA also attenuated social preference for the stranger mouse (M2) and total sniffing time with both mice (Fig. 6i-k). Moreover, pretreatment with haloperidol in the VTA impaired social interaction with a stranger intruder mouse in the home-cage social test (Fig. 6l). Therefore, systemic treatment with haloperidol or other antipsychotic agents targeting D2Rs may inadvertently exacerbate schizophrenia-associated symptoms by promoting the over-excitation of DA neurons and thus the increased DA release. These results further support a role of DA over-transmission during adolescence in the pathogenesis of schizophrenia.
D2R as a dual therapeutic target for the treatment of schizophrenia
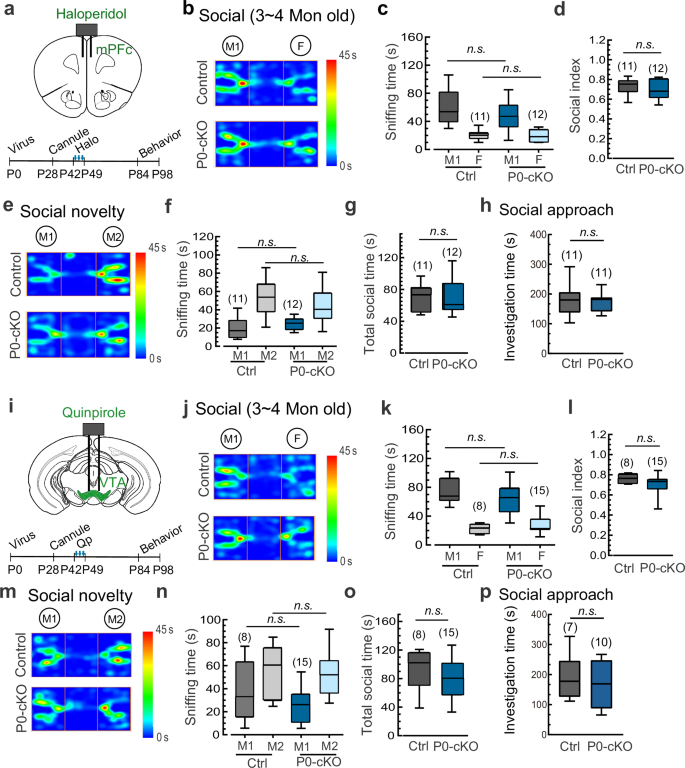
Considering the opposing roles of presynaptic and postsynaptic D2Rs in DA transmission, we propose that local administration of haloperidol or other D2R antagonists in the mPFC could be an effective approach to alleviate negative symptoms by rectifying the aberrant DA transmission postsynaptically (with a mixed effect, but the postsynaptic effect predominated as shown in Fig. 6d). To test this hypothesis, we locally infused haloperidol into the mPFC during the period of P42–P56 and monitored its impact on the social behaviors of Syt11 P0-cKO mice (Fig. 7a). Notably, a single administration of haloperidol into the mPFC restored the decreased social interaction with the M1 mouse and the reduced social preference index in Syt11 P0-cKO mice (Fig. 7b, c). Similarly, the diminished social preference for the stranger mouse (M2) and the total sniffing time with both mice were completely attenuated (Fig. 7d, e). Furthermore, compared with the impaired social interaction observed in saline-control Syt11 P0-cKO mice, the haloperidol-treated cKO mice exhibited a social interaction time indistinguishable from that of control mice in the home-cage social test (Fig. 7f). Therefore, local application of a D2R antagonist into the mPFC before late adolescence (or early adulthood) can rescue the schizophrenia-like social deficits. These findings not only provide a reasonable explanation for the limited effectiveness of well-known antipsychotics in alleviating negative symptoms in schizophrenia, but also propose a potential therapeutic strategy for the clinical treatment of the disease.
a Schematic of bilateral cannula application of the D2R antagonist haloperidol (Halo, 50 μM) into the mPFC of juvenile Syt11 P0-cKO or control mice (TH-Cre-EGFP or TH-EGFP AAV injected into the VTA of neonatal Syt11 flox/flox mice) and the experimental procedure. b, c Statistics of the three-chamber social interaction test in Syt11 P0-cKO and control mice following local application of Halo vs saline in the mPFC. d, e Statistics of the social novelty test in Halo- vs saline-treated Syt11 P0-cKO and control mice. f Statistics of investigation time in Halo- vs saline-treated Syt11 P0-cKO and control mice in the home-cage social test. g Schematic of bilateral cannula application of the D2R agonist qunipirole (Qp, 1 μg/μl) into the VTA of juvenile Syt11 P0-cKO and control mice and the experimental procedure. h, i Statistics of the three-chamber social interaction test in Syt11 P0-cKO and control mice following local application of Qp vs saline in the VTA. j, k Statistics of the social novelty test in Qp- vs saline-treated Syt11 P0-cKO and control mice. l Statistics of investigation time in Qp- vs saline-treated Syt11 P0-cKO and control mice in the home-cage social test. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s post-hoc test, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
The present study has provided direct in vivo evidence that DA over-transmission during preadolescence is a risk factor in initiating the pathogenesis of schizophrenia (Figs. 1–4). Therefore, it is plausible to reverse the social deficits by locally delivering a D2R agonist into the VTA, which inhibits DA neurons by targeting somatic D2Rs, to rectify the hyperactivity of DA neurons during this period. Similar to the effects of haloperidol in the mPFC, local delivery of the D2R agonist quinpirole into the VTA also demonstrated a therapeutic effect in adolescent Syt11 P0-cKO mice (Fig. 7g). Specifically, compared to the impaired social preference of Syt11 P0-cKO mice injected with saline, quinpirole-treated P0-cKO mice showed intact social interaction with the M1 mouse and an unchanged social preference index (Fig. 7h, i). In addition, local application of quinpirole in the VTA fully reversed the pronounced social withdrawal of Syt11 P0-cKO mice in the social novelty test (Fig. 7j, k). Moreover, social interaction with the stranger intruder mouse in the home-cage social test was substantially attenuated by the local administration of quinpirole (Fig. 7l).
To further validate the therapeutic effect of quinpirole in the VTA, we utilized dizocilpine (MK-801)-induced schizophrenia-like mice. Consistent with our hypothesis, pre-treatment with MK-801 indeed increased DA overflow in mPFC slices (Supplementary information, Fig. S18a, b), while MK-801 administration during preadolescence led to long-lasting social behavior deficits, which were fully reversed by the local application of quinpirole in the VTA (Supplementary information, Fig. S18c). Importantly, local application of quinpirole in the VTA to block the elevated DA release or haloperidol in the NAc to diminish the enhanced DA transmission to post-synaptic D2R neurons reversed sensory gating dysfunction in the MK801-induced schizophrenia mouse model (Supplementary information, Fig. S18d). These results further confirm the role of DA over-transmission before late adolescence in the development of schizophrenia and propose a therapeutic strategy targeting D2Rs for the clinical treatment of the disorders. Collectively, the present work suggests that both the presynaptic/somatic application of D2R agonists in the VTA and the postsynaptic delivery of D2R antagonists in the mPFC represent promising therapeutic approaches for the clinical treatment of schizophrenia-associated social withdrawal.
Long-lasting rescue of social withdrawal by targeting presynaptic or postsynaptic D2Rs
We have successfully demonstrated that the rectification of DA transmission via the brain region-specific intervention of D2Rs during adolescence can effectively reverse social deficits in schizophrenia. To investigate the long-lasting effects of this therapeutic approach, we conducted repeated local administration of haloperidol in the mPFC every second day from P42 to P49. Notably, we observed sustained rescue effects on social behaviors in Syt11 P0-cKO mice (Fig. 8a-h). Specifically, the adult haloperidol-treated P0-cKO mice showed normal sniffing time with a stranger M1 mouse and an unchanged social preference index in the three-chamber social test, similar to control mice (Fig. 8b-d). In addition, they exhibited intact social preference for the stranger M2 mouse and normal total social time with both mice in the social novelty test (Fig. 8e-g). Consistent with these results, the social interaction time with a stranger intruder mouse was effectively restored in adult haloperidol-treated P0-cKO mice in the social approach test (Fig. 8h). These findings confirm a complete and long-lasting recovery of social deficits through the suppression of elevated DA transmission via local application of a D2R antagonist in the mPFC.
a Schematic of repetitive bilateral cannula application of a D2R antagonist (Halo) in the mPFC (every second day during P42-P49) and the experimental procedure for assessing social behaviors in Syt11 P0-cKO vs control mice. b–d Representative heat maps and statistics of three-chamber social interaction in adult (3–4 months) Halo-treated Syt11 P0-cKO vs control mice as described in a. e–g Representative heat maps and statistics of the three-chamber social novelty test in adult Halo-treated Syt11 P0-cKO vs control mice. h Statistics of investigation time of adult Halo-treated Syt11 P0-cKO vs control mice in the social approach test. i Schematic of repetitive bilateral cannula application of a D2R agonist (Qp) in the mPFC (every second day during P42-P49) and the experimental procedure for assessing social behaviors in Syt11 P0-cKO vs control mice. j–l Representative heat maps and statistics of three-chamber social interaction test of adult (3–4 months) Qp-treated Syt11 P0-cKO vs control mice as described in i. m–o Representative heat maps and statistics of the three-chamber social novelty test of adult Qp-treated Syt11 P0-cKO vs control mice. p Statistics of investigation time of adult Qp-treated Syt11 P0-cKO vs control mice in the social approach test. Data are shown as box-and-whisker plots, with the median represented by the central line inside each box, the 25th and 75th percentiles represented by the edges of the box, and the whiskers extending to the most extreme data points. Ordinary two-way ANOVA followed by Bonferroni’s post-hoc test (c, f, k, n) or two-tailed Mann-Whitney test (d, g, h, l, o, p), *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significant difference. Source data are provided as a Source Data file.
Next, we determined whether local delivery of a D2R agonist into the VTA during adolescence could also produce long-lasting rescue effects on social deficits in Syt11 P0-cKO mice (Fig. 8i). Interestingly, similar to the haloperidol treatment in the mPFC, repeated local treatment with the D2R agonist quinpirole into the VTA restored the impaired social interaction with the stranger M1 mouse and the reduced social preference index in the three-chamber test in Syt11 P0-cKO mice (Fig. 8j-l). Furthermore, the social preference for the M2 mouse and the total social time with both mice were indistinguishable between adult quinpirole-treated Syt11 P0-cKO mice and control mice (Fig. 8m-o). Finally, the social approach test confirmed that the therapeutic effects of quinpirole on social withdrawal in Syt11 P0-cKO mice were maintained into adulthood (Fig. 8p). Taken together, these findings provide valuable insights into long-lasting therapeutic strategies for schizophrenia by targeting D2Rs either presynaptically or postsynaptically before late adolescence, offering potential benefit for the permanent recovery of schizophrenia in clinical treatment.