This study was conducted at the Mallinckrodt Institute of Radiology (MIR) PET facility at Washington University School of Medicine (WUSM) in St. Louis during 2020 and was approved by the WUSM Institutional Review Board in accordance with ethical standards established in the 1964 Declaration of Helsinki. Figure S1 depicts a schematic of the study design.
Upon providing informed consent, participants were screened to confirm eligibility within 14 days of baseline PET (inclusion/exclusion criteria in Supplement Information). Safety monitoring included vital signs (blood pressure and heart rate, oral body temperature, and respiratory rate), 12-lead electrocardiograms (ECGs), clinical laboratory testing (hematology, clinical chemistry, and urinalysis), adverse event (AE) assessments, and physical examination. The Columbia-Suicide Severity Rating Scale (C-SSRS) was used to assess suicide risk. The overall schedule of assessments for the study are outlined in Table S1.
Enrollment
Twenty-four volunteers were screened and sixteen were enrolled based on inclusion/exclusion criteria. All enrolled participants completed the study and were included in the analyses. Sixteen healthy volunteers (7 female, 9 male) ages 18 to 55 years and with a body mass index (BMI) between 18 and 30 kg/m2 were enrolled in the study. No enrolled subjects dropped out of the study. Sizing of this study was based on prior experience with PET imaging studies and did not take statistical powering assumptions into account. Table 1 summarizes demographic data of study participants.
Study design
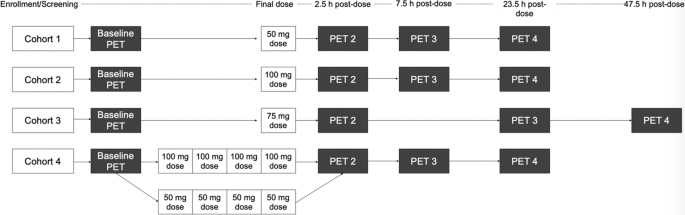
This was an open label study, adaptive design, study. Cohort 1 was chosen to be a 50 mg single oral dose based on prior preclinical and human PK non-PET imaging studies [13]. The 75 mg and 100 mg doses were determined empirically after observation of prior doses and were determined empirically with the goal of bracketing 60–80% dopamine RO. Timing of PET scans, starting at 2.5, 7.5, and 23.5 h were selected for the 50 and 100 mg single doses and multiple doses to capture dopamine RO for a full 24 h period after dosing. In the 75 mg single dose arm the 7.5 h scan was replaced by a scan starting at 47.5 h to better understand the decay kinetics of dopamine RO resulting from LB-102. This additional time point did not affect interpretation of the results beyond reassuring that dopamine RO would not persist indefinitely.
On providing informed consent, subjects were screened to determine study eligibility within 14 days of baseline PET scan. Within two weeks after baseline PET, subjects checked in to the WashU Center for Translational Research Unit (CTRU) to receive their first dose of LB-102. This inpatient stay allowed for frequent medical monitoring after dosing and after PET scans.
Subjects in Cohorts 1 to 3 received single doses and subjects in Cohort 4 received once daily doses over 4 days—a Phase 1 clinical study of LB-102 [14] demonstrated that LB-102 plasma concentration reached steady state the morning of dosing day 4. The 4-day dosing was designed to capture receptor occupancy under steady state conditions.
In Cohorts 1-3, a single dose of LB-102 was administered orally as a powder in capsule on day 1 of the study and in Cohort 4 once a day for four days. Dose administration was scheduled for 8:00 AM (±1 h) each dosing day, following the collection of vital signs and a 12-lead ECG. The dose was given with water and subjects had the option to eat breakfast after dosing. All cohorts underwent four PET scans, including one baseline scan prior to administration of LB-102 followed by three post-dose scans at varying timepoints. In cohorts 1 and 2, post-dose scans started at 2.5 h, 7.5 h, and 23.5 h after dosing. For cohort 3, post-dose scans started at 2.5 h, 23.5 h, and 47.5 h after dosing. Subjects in Cohort 4 underwent post-dose scans following the final dose (day 4) of LB-102 at 2.5 h, 7.5 h, and 23.5 h after final dosing. PET scans were acquired over 90 min and values reported are a time-weighted average of values obtained. Subjects were discharged from the CTRU following their final PET scan. A schematic of the trial design is presented in Fig. 2.
In Cohort 1, all four subjects were dosed with 50 mg LB-102. In Cohort 2, all subjects were dosed with 100 mg LB-102, and in Cohort 3 all subjects were dosed with 75 mg LB-102. Initially, all four subjects in Cohort 4 were to receive 100 mg LB-102; the first two subjects demonstrated higher than expected dopamine receptor occupancy at 100 mg QD for four days, approaching 90%. It was decided to reduce dosing to 50 mg for the final two subjects out of an abundance of caution.
Radiotracer and positron emission tomography
Subjects received a total of 4 PET scans with 11C raclopride as a tracer. The mean injected activity was 14.1 ± 0.31 mCi (SEM). One baseline PET scan (pre-dose) and 3 PET scans were obtained at several time points following final LB-102 dose. Each PET scan lasted 90 min and collected 30 frames (four 15 s, four 30 s, three 1 min, two 2 min, five 4 min, and twelve 5 min). PET scans were obtained on a PET-CT Siemens Vision and MRI on a 3 T Prisma. A T1-weighted 3D MPRAGE MRI sequence was acquired on each subject using the following parameters: repetition time, 2400 ms; echo time, 2.62 ms; flip angle, 8; slice thickness, 1 mm; field of view, 256 mm × 256 mm; number of axial slices, 176.
Image processing and derivation of PET outcome
PET images were analyzed using PMOD [21]. T1-weighted MRI was used for co-registration of PET images. Images were normalized into standard space. Volumes of interest (VOIs) from Hammers template [22] (caudate, putamen, thalamus, temporal lobe, and cerebellum) were applied to PET frames to obtain regional time-activity curves (TACs). TACs were used for tracer kinetic modeling and binding potential non-displaceable (BPND) was computed at caudate, putamen, thalamus, and temporal lobe taking cerebellum as a reference region. In PMOD, kinetic modeling analyses were performed using the simplified reference tissue model (SRTM) [23] and Logan [24] graphical reference tissue model.
RO was obtained for each of the 3 PET scans (scans 2, 3, or 4) following the baseline PET scan as follows:
$${RO}=\,100\,X\frac{{{BP}}_{{ND}}{{Baseline\; scan}}_{i}{{Region}}_{j}-{{BP}}_{{ND}}{{postdose\; scan}}_{i}{{Region}}_{j}}{{{BP}}_{{ND}}{{Baseline\; scan}}_{i}{{Region}}_{j}} \%$$
Where the i = 2nd, 3rd, or 4th PET scan and region j is the caudate, putamen or thalamus region.
To assess the reproducibility of results from above two methods, the RTGA 10T60, RTGA 10T90 [25], and MTRM2 [26] were further used using IDEA [27]. In IDAE, VOIs were determined using Freesurfer [28]. Cerebellum VOIs were manually edited to exclude non-cerebellar elements include local sinus.
11C raclopride was produced at the MIR cyclotron facility at high specific activity (940.41 ± 35.40 Ci/mmol) and average injected mass (5.42 ± 0.14 µg). Subjects all had head fixation with an individualized thermoplastic mask. All PET scans were carried out after a short bolus injection of the radiotracer followed by dynamic PET imaging of 30 frames over 90 min on a Siemens PET CT Vision where the attenuation scan was carried out first by a short non contrast CT scan. Reconstruction employed an iterative 3D time of flight (True X + TOF (Ultra HD) 8 iterations, 5 subsets, Allpass Filter) and dynamic scan durations were four 15 s, four 30 s, three 60 s, two 120 s, five 240 s, twelve 300 s starting with the IV injection of the radiotracer.
Each subject received a baseline PET in the absence of LB-102, then repeat PET scans according to the Study Design described above with up to three post single dose (cohorts 1 -3) and after four days of chronic dosing as inpatients in the WUSTL CTRU (Center for Translational Research Unit).
Safety
Safety was monitored during the study using the following procedures and assessments at regular intervals: blood pressure, heart rate, oral body temperature, 12-lead ECGs, clinical labs (hematology, clinical chemistry, and urinalysis), adverse event (AE) assessments, and physical examinations. Subjects were given the Columbia-Suicide Severity Rating Scale (C-SSRS) to assess suicide risk. Follow-up by telephone was conducted on Day 3 for subjects receiving single doses and on Day 7 for subjects receiving multiple doses.
Data analysis
LB-102 RO was determined as the amount of 11C raclopride displaced by LB-102 using PET at baseline (pre-dose Day 1) and starting at 2.5 h, 7.5 h, and 23.5 h for Cohorts 1 and 2, at baseline and starting at 2.5 h, 23.5 h, and 47.5 h for Cohort 3, and at baseline and at 74.5 h, 79.5 h, and 95.5 h for Cohort 4 in the caudate, putamen, thalamus, and temporal cortex.